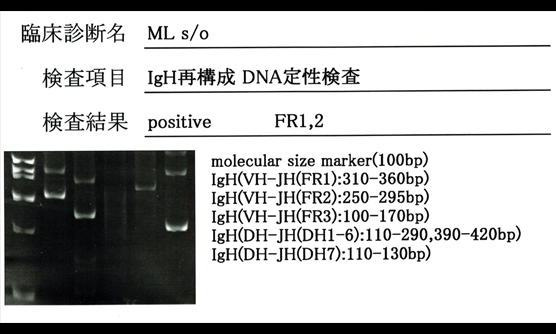
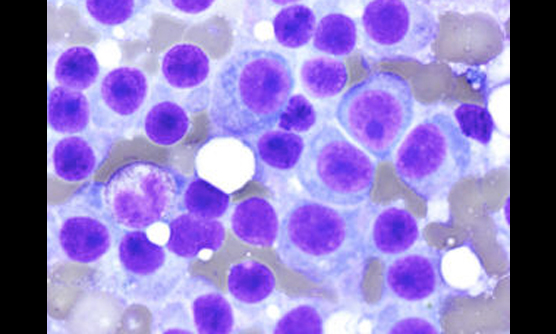
血液内科は、溶血性貧血、巨赤芽球性貧血、再生不良性貧血、特発性血小板減少性紫斑病などの良性血液疾患と、急性白血病、骨髄異形成症候群、悪性リンパ腫、多発性骨髄腫などの造血器悪性腫瘍を担当します。当院は救命救急センターであり、重症再生不良性貧血や血栓性血小板減少性紫斑病など迅速診断と速やかな専門的治療を要する稀少血液疾患患者が、神戸市内はもとより兵庫県全域から紹介されてきます。また、本院の「断らない救急」の理念に沿って、診療所や他病院からの転送はすべて受け入れています。
急性白血病をはじめとした血液疾患の多くは、診断や治療において速やかかつ適切な対応が求められます。そのためには経験・知識とともに、気力・体力が求められます。2022年5月現在、当科には日本血液学会専門医の資格を持つ7名の中堅・ベテラン医師と、7名の血液内科専攻医が在籍しています。中堅・ベテラン医師3名と若手医師3-4名がチームを組み、十分な議論を行うことで、常にベストな対応を行っています。患者さんからみると、主治医のほかに多くの担当医がいることになりますが、夜間休日だけでなく、主治医の体調不良時や学会開催中においても、安全かつ丁寧な医療を途切れることなく受けられることができます。血液内科当番医は毎日在院しており、休日に緊急入院された患者さんに対しても最善の医療が行われています。
近年造血器腫瘍の治療に多くの分子標的薬剤が用いられるようになりました。特に悪性リンパ腫や急性リンパ性白血病に対してCAR-T療法という画期的な治療法が導入されました。当院では2020年11月にCAR-T療法であるキムリア®の施設認定を受け、その後ブレヤンジ®の認定も受けました。本年になって多発性骨髄腫に対するCAR-T療法が保険収載され、CAR-T療法のさらなる適応拡大がみられています。当院では2022年5月までに12名の患者にCAR-T治療を行いましたが、兵庫県内でCAR-T療法が施行可能施設はわずかであり、今後ますます多くの患者さんにCAR-T療法を提供すべく努力していきます。
当科の特徴の一つに、多くの新規薬剤の開発治験にもかかわっていることが挙げられます。治療に難渋している患者さんに対しても、期待の持てる治療を治験として提供できる可能性があります。また、治験での経験を通じて、新規薬剤の発売直後から適正かつ積極的に治療に取り入れていくことができます。
血液疾患を患った神戸市民、兵庫県民のため、世界水準の治療を安全かつ確実にお届けすること、それが私どもの使命です。
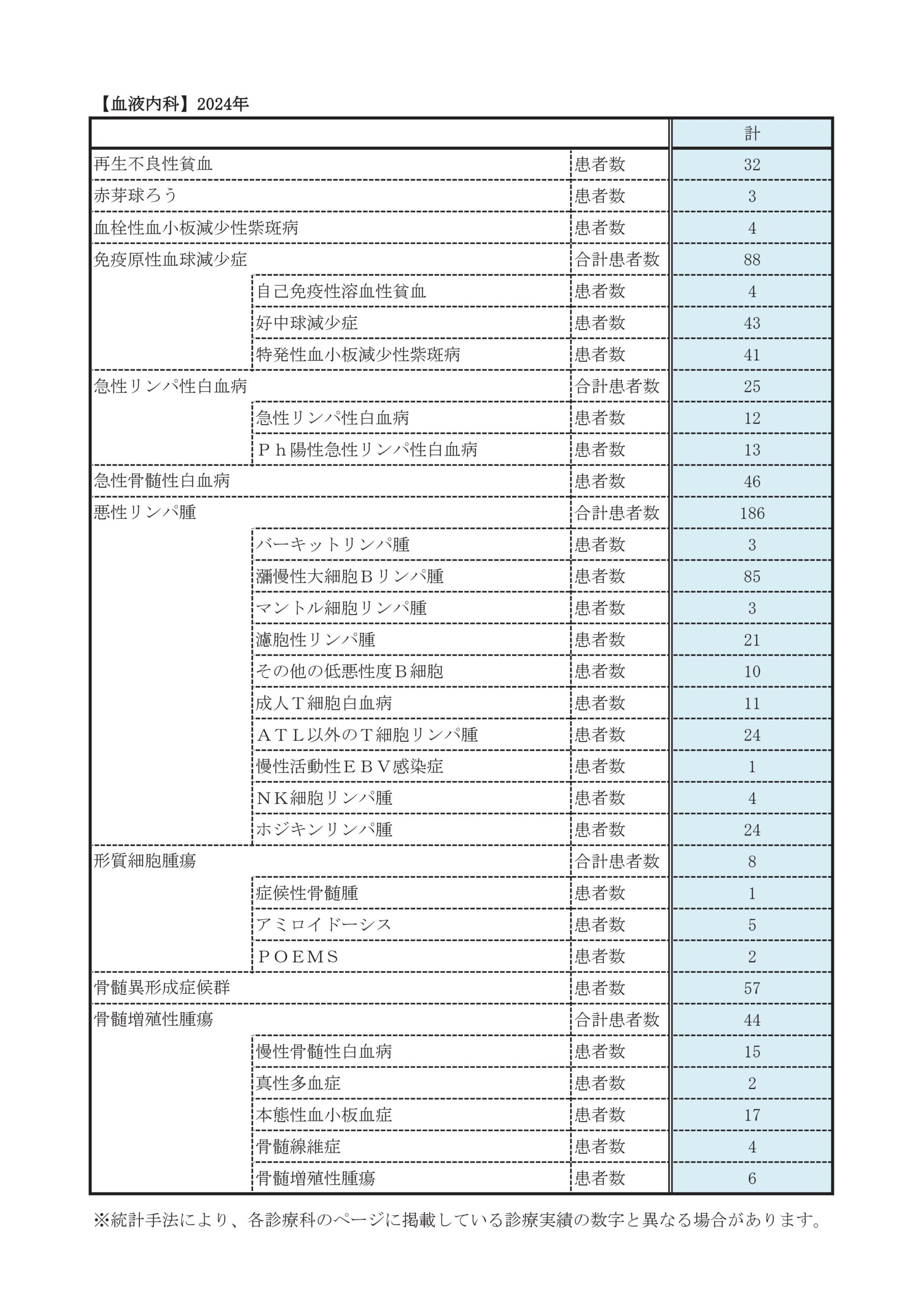
診療実績
年次患者数
※2020年は新型コロナウイルス感染症の影響で減少しています。
| 初診患者数 | ||||||||
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|---|---|---|
| 再生不良性貧血 | 8 | 12 | 13 | 11 | 6 | 8 | 2 | 7 |
| 赤芽球ろう | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| 血栓性血小板減少性紫斑病/非定形的HUS | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 2 |
| 免疫原性血球減少症 | 22 | 27 | 18 | 37 | 38 | 18 | 20 | 13 |
| 自己免疫性溶血性貧血/好中球減少症 | 4 | 5 | 7 | 5 | 7 | 1 | 5 | 6 |
| 特発性血小板減少性紫斑病 | 18 | 25 | 12 | 32 | 31 | 17 | 15 | 7 |
| 急性リンパ性白血病 | 13 | 10 | 17 | 15 | 10 | 10 | 12 | 11 |
| 前駆B細胞 | 2 | 4 | 5 | 5 | 4 | 4 | 2 | 2 |
| Ph陽性 | 7 | 4 | 7 | 5 | 5 | 4 | 6 | 6 |
| 前駆T細胞 | 2 | 2 | 4 | 5 | 1 | 1 | 2 | 2 |
| 混合型/未分化型 | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 1 |
| 急性骨髄性白血病 | 31 | 38 | 40 | 52 | 34 | 25 | 42 | 53 |
| 新規発症 | 29 | 33 | 36 | 45 | 31 | 24 | 37 | 45 |
| 二次性(MDS/MPN由来) | 1 | 5 | 2 | 2 | 3 | 1 | 2 | 1 |
| 治療関連 | 1 | 0 | 2 | 5 | 0 | 0 | 3 | 7 |
| 悪性リンパ腫 | 151 | 172 | 131 | 137 | 167 | 133 | 148 | 143 |
| バーキットリンパ腫 | 1 | 3 | 3 | 1 | 2 | 2 | 2 | 1 |
| 瀰漫性大細胞Bリンパ腫 | 76 | 77 | 64 | 75 | 81 | 56 | 73 | 89 |
| マントル細胞リンパ腫 | 2 | 5 | 2 | 2 | 5 | 6 | 1 | 2 |
| 濾胞性リンパ腫 | 44 | 31 | 13 | 21 | 28 | 26 | 20 | 16 |
| その他の低悪性度B細胞 | 18 | 18 | 21 | 27 | 21 | 24 | 15 | |
| 成人T細胞白血病 | 6 | 7 | 5 | 2 | 5 | 5 | 2 | 2 |
| ATL以外のT細胞リンパ腫 | 14 | 15 | 12 | 11 | 11 | 8 | 16 | 6 |
| 慢性活動性EBV感染症 | 2 | 2 | 0 | 2 | 0 | 0 | 1 | |
| NK細胞リンパ腫 | 1 | 4 | 4 | 1 | 1 | 2 | 3 | 2 |
| ホジキンリンパ腫 | 7 | 10 | 8 | 3 | 5 | 5 | 7 | 9 |
| 形質細胞腫瘍 | 31 | 36 | 27 | 30 | 26 | 20 | 22 | 13 |
| 症候性骨髄腫 | 28 | 34 | 22 | 24 | 24 | 19 | 22 | 10 |
| アミロイドーシス | 3 | 2 | 5 | 5 | 2 | 1 | 0 | 3 |
| POEMS | 1 | 0 | 0 | 0 | 0 | |||
| 骨髄異形成症候群 | 24 | 21 | 25 | 33 | 33 | 17 | 15 | 10 |
| 低リスク群 | 7 | 5 | 14 | 22 | 14 | 5 | 9 | 5 |
| 高リスク群 | 17 | 16 | 11 | 11 | 19 | 12 | 6 | 5 |
| 骨髄増殖性腫瘍 | 22 | 28 | 16 | 28 | 35 | 23 | 22 | 19 |
| 慢性骨髄性白血病 | 10 | 10 | 9 | 8 | 3 | 4 | 3 | 4 |
| 真性多血症 | 5 | 6 | 1 | 6 | 5 | 5 | 2 | 4 |
| 本態性血小板血症 | 3 | 4 | 1 | 11 | 23 | 8 | 8 | 5 |
| 骨髄線維症 | 0 | 5 | 3 | 0 | 0 | 2 | 3 | 1 |
| MDS/MPN | 4 | 3 | 2 | 3 | 4 | 4 | 6 | 5 |
移植件数
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| 同種造血幹細胞移植 | 45 | 43 | 37 | 37 | 38 | 28 | 33 | 27 |
|---|---|---|---|---|---|---|---|---|
| 血縁 | 10 | 8 | 17 | 13 | 23 | 19 | 18 | 9 |
| 非血縁 | 16 | 10 | 7 | 13 | 7 | 2 | 10 | 8 |
| 臍帯血 | 19 | 25 | 13 | 11 | 8 | 7 | 5 | 10 |
| 自家造血幹細胞移植 | 19 | 31 | 18 | 20 | 13 | 22 | 14 | 13 |
| 移植件数総計 | 64 | 74 | 55 | 57 | 51 | 50 | 47 | 40 |
| CAR-T療法 | 9 | 7 |
学術活動
2024年学術報告 2023年学術報告 2022年学術報告 2021年学術報告 2020年学術報告 2019年学術報告 2018年学術報告 2017年学術報告 2016年学術報告 2015年学術報告 2014年学術報告 2013年学術報告 2012年学術報告診療科別統計
主な疾患・治療法
当科は凝固異常症を含めて血液疾患全般を対象に診療を行っていますが、なかでも頻度の高い以下の疾患と同種造血幹細胞移植に対する当科での取り組みを記します。

完全寛解になったのちにも、治癒を目指して治療が続けられますが、その際数回の化学療法を行えばよい患者さんと、化学療法だけでなく同種造血幹細胞移植が必要な患者さんに分かれます。同種造血幹細胞移植は長期生存が望める確率が高い反面、治療そのものの併発症により生命に危険を生じえます。治療開始後早期に、同種造血幹細胞移植の必要性を見極めることが重要です。
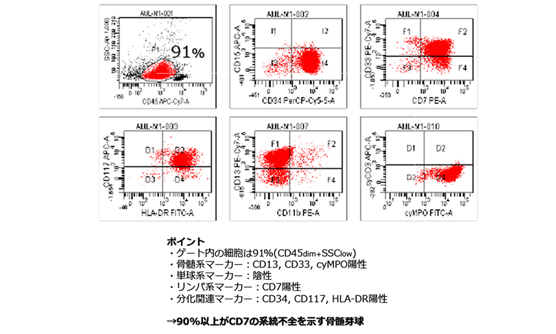
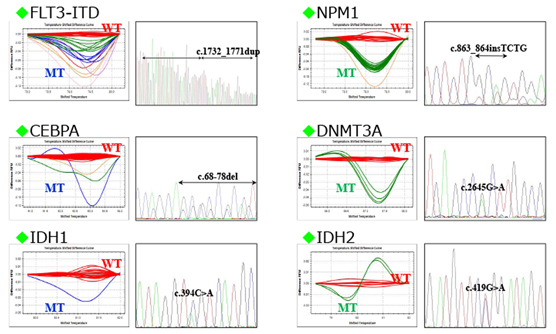
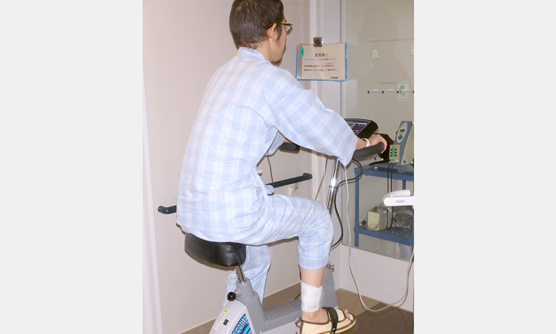
当院では、院内の遺伝子検査室で,白血病の予後に影響する変異遺伝子や、多重フローサイトメトリー法や高感度遺伝子解析で残存白血病細胞量を経時的に調べ、同種造血幹細胞移植の適応を決めています。また、当院では白血病の治療中でクリーンルームに入っている患者さんにも、理学療法士によるがんリハビリテーションを行うことで、治療の継続や社会生活へのスムーズな復帰に必要な体力維持の取り組みを行っています。
これらの努力により予後不良の遺伝子異常をもたない患者の治療成績は向上しています。
「急性骨髄性白血病の治療成績」はこちらから
子供に多い病気で、内科領域でも40歳以下の患者さんが多いです。25歳以下では小児科で行われてきた化学療法を行い、それ以上の患者さんにはHyperCVAD/HD-MAという内科領域で編み出された治療法を主に行っています。
内科領域ではフィラデルフィア染色体(Ph)陽性の患者さんが多く(約4割)、そのような患者さんにはチロシンキナーゼ阻害剤を併用しています。化学療法だけの場合は2年間にわたる維持強化療法を行いますが、Ph陽性患者さんでは積極的に同種造血幹細胞移植を行います。また、それ以外の患者さんでも、遺伝子解析を用いての微少残存腫瘍量の検討により同種移植の適応を決めています。化学療法により残存腫瘍量を可能な限り減らすことが、化学療法においても同種移植においても最も重要です。
「急性リンパ性白血病の治療成績」はこちらから
21世紀になってリツキシマブやベンダムスチンなどの新規薬剤が導入され、B細胞性リンパ腫においては治療成績の大幅な改善が得られました。今後も多くの新規薬剤の導入が期待されています。最も頻度の高い病型であるび漫性大細胞B細胞リンパ腫の5年生存率は68%に達しています。
「びまん性大細胞B細胞リンパ腫の治療成績」はこちらから進行の遅い低悪性度B細胞リンパ腫においては、病気の治癒を求めるのでなく、病気と共存しつつ長生きするという方針も現実的な選択肢となりました。
「濾胞性リンパ腫の治療成績」はこちらから一方、末梢性T細胞リンパ腫や成人T細胞白血病では、化学療法単独での成績は十分でなく、自家もしくは同種造血幹細胞移植も考慮されます。いずれにせよ、悪性リンパ腫の治療においては、新しい考えに基づいた病理分類や、それを補完するための細胞遺伝学的な検査は不可欠です。当科ではすべての悪性リンパ腫患者さんの治療に際して、病理学的、細胞遺伝学的検討結果を踏まえて、短期ならびに中長期的な治療方針を決めています。
用いられる薬剤は大きく分けて6種類です。すなわち、ステロイド剤、アルキル化剤(メルファラン、シクロフォスファミドなど)、プロテオゾーム阻害薬(ボルテゾミブ、カーフェルゾミブ、イキサゾミブ)、IMiDsと呼ばれる免疫調節薬(サリドマイド、レナリドミド、ポマリドミド)、抗体製剤(エロツズマブ、ダラツムマブ)、及びその他(パノピノスタット、ドキソルビシンなど)です。これら6種類の薬のうち2〜3種類を組み合わせて使うのが一般的ですが、ステロイド剤とアルキル化剤、ドキソルビシン以外はすべて2006年以降に発売になった薬剤で、特に2015年から2017年にかけて6つの薬剤が発売になりました。どのような組み合わせがよいのか、どのような順番で使えばよいのかは現在世界中で検証中です。実際には、薬同士の相互作用が確認されていない組み合わせも多くありますので、安全性が確認されている薬の組み合わせ、10種類程度、の中から実際の治療法を選択することとなります。
治療の目標は自覚症状のない長期の生存です。その延長として治癒に至ることを期待します。自覚症状のない長期の生存のためには骨髄腫の腫瘍量を免疫固定法やフリーライトチェイン法が陰性もしくは正常化する程度まで減らすことが望ましいと考えられています。具体的には、初回治療には3剤の併用療法を行い、引き続いて70歳以下の患者さんには大量化学療法を併用した自家造血幹細胞移植を行う、さらに年単位での維持療法を行うことが一般的ですが、患者さんの病状に即して最良の治療選択を行うことを目指しています。本邦未発売の新規薬剤の治験にも多く参加していますので、お気軽にお問い合わせください。
高リスク群骨髄異形成症候群では、白血病へ移行するのを防ぐ目的で急性骨髄性白血病に準じた化学療法やアザシチジン治療を行います。低リスク群、高リスク群ともに病気を治すためには同種造血幹細胞移植が必要です。特に高リスク群においては積極的に同種造血幹細胞移植を行なうことにしています。

同種造血幹細胞移植を行うためには造血幹細胞のドナーが必要です。ドナー候補としては同胞や子供などの患者さんの親族、骨髄バンクの非血縁ドナー、ならびに臍帯血バンクの保存臍帯血が挙げられます。患者さんの親族間でのドナー検索においては、患者さんやドナー候補者のプライバシーを守ることが重要であり、当院では日本造血細胞移植学会認定造血細胞移植コーディネーターがこの役にあたっています。また、移植を受けた患者さんは、退院後にも種々の合併症が起きえますし、生活の注意点や若干の制限が必要になる場合があります。
移植後の日常生活に関する疑問を解決すること、より快適な生活を送れるようにすること、体調の自己管理が容易にできること、このようなことを目的として日本造血細胞移植学会の研修を受けた看護師による移植後長期フォローアップ外来も行っております。
「同種造血幹細胞移植の成績」はこちらから
CAR-T細胞療法は「キメラ抗原受容体(CAR)」を導入して、がん細胞などを攻撃するように作り替えたT細胞を用いた治療です。患者さんからT細胞を採取して、急性リンパ性白血病などが持つ「CD19」というたんぱく質を標的としたCAR-T細胞を作成し、患者さんに戻すのがキムリア®です。
「CAR-T細胞療法(キムリア®)」について詳しくはこちら臨床研究
現在施行中の臨床研究と治験
臨床研究、治験とは?
臨床研究とは、人を対象としておこなわれる医学研究のことを言います。病気の予防、診断、治療法だけでなく、病気の原因の解明、患者さんの生活の質(QOL)の向上を目的としておこなわれます。治験も臨床試験も臨床研究の一つです。
臨床試験とは、臨床研究のうち、薬剤、治療法、診断法、予防法などの安全性と有効性を評価することを目的にしておこなわれる臨床研究です。
治験とは、新しい薬や医療機器が一般的の診療で使用できるように、国の承認を得るためにおこなわれる臨床試験です。 既存の診療情報を用いて(研究の目的で検査等を追加でおこなうこともあります)、病気の経過、おこなわれた診療の効果や影響を研究する、観察研究という臨床研究もあります。
当科では、院内の倫理委員会の承認を受け、治験、臨床試験を含むさまざまな臨床研究をおこなっています。
治験
治験には、有効な治療法があまりない患者さんに新しい薬が使える、また有効性が認められているが国内では承認されていない薬を使うことができるというメリットがありますが、副作用などが十分に知られていないというデメリットがあります。十分に医師、治験コーディネーターの話を聞いていただき、治験に参加するかどうかを決めるようにしてください。もちろん、一度いただいた同意を撤回していただくことも自由です。
血液内科で施行中の治験に関する情報はこちらに載せております。臨床試験(介入研究)
臨床試験は、患者さんに、研究を目的とした何らかの検査や治療、ケアを受けていただき、その有効性や影響を評価する研究です。当科で以下のような臨床研究をおこなっています。これらの研究に適格性がある場合は、研究の参加をお願いすることがあります。治験同様、研究への参加は自由意志であり、一度いただいた同意を撤回していただくことも自由です。
血液内科で現在実施中の臨床研究
| 研究課題名 | 研究終了予定 | 説明文PDF |
| 市中病院における抗CD-19 CAR-T細胞療法を受けた患者52例の治療成績に関する単一施設後方視検討 | 2026/3/31 | |
|---|---|---|
| 自己免疫性溶血性貧血におけるフローサイトメトリー法による赤血球膜結合IgGと補体の定量とIgGサブクラスの解析 | 2031/3/31 | |
| 多施設共同骨髄系腫瘍レジストリ研究 (MYKURE:Myeloid Malignancy Kyoto University Registry) |
2030/3/31 | |
| 同種造血幹細胞移植後の類洞閉塞症候群に対するDefibrotideの有効性・安全性の検討 | 2026/3/31 | |
| 京都造血幹細胞移植グループの造血幹細胞移植データを用いた移植成績の解析 | 2027/4/16 | |
| T細胞性リンパ腫発症機序の解明 | 2027/3/31 | |
| 造血器腫瘍における遺伝子異常の網羅的解析 | 2029/3/31 | |
| 造血細胞移植および細胞治療の全国調査 | 未定 | |
| 疫学調査「血液疾患登録」 | 2031/12/31 | |
| 日本における骨髄増殖性腫瘍の予後に関する大規模多施設前向き観察研究 | 2031/3/31 | |
| 新規自動血球分析システムの分析性能の検証研究 | 2026/12/31 | |
| 濾胞性リンパ腫におけるobinutuzumabの効果・耐性に関わる臨床分子病理学的検討 | 2026/9/30 | |
| キメラ抗原受容体(CAR)T 細胞療法における製造効率に関する検討 | 2026/3/31 | |
| OCV-501の第II相臨床試験の予後追跡調査試験(OCV-501 長期観察研究) | 2022/3/31 |
既存の診療情報をもちいた観察研究
当科では、既存の診療情報、つまり、日常診療で得られた情報を利用して、病気の経過や診療の効果、影響について検討する研究(観察研究)もおこなっています。観察研究は、上記の治験や臨床試験とは異なり、直接文書による同意はいただかず、このホームページ上での掲示によるお知らせを持って同意をいただいたものとしておこなわれます。研究の趣旨についてご理解いただき、ご協力を賜りますようお願い申し上げます。
現在、当科では、下記の観察研究をおこなっています。研究内容については、情報公開文書(PDFファイル)を御覧ください。研究計画や内容についてより詳しくお知りになりたい方、ご自身の診療情報がこれらの研究で利用されることについて異議のある方、その他ご質問がある方は、下記の連絡先へお問い合わせ下さい。
血液内科で現在実施中の観察研究
| 研究課題名 | 院内の研究責任者 | 情報公開文書 |
| チサゲンレクルユーセルの規格外品の安全性と有効性を検討する観察研究 | 永井 雄也 | PDF 別紙 |
|---|---|---|
| 造血器腫瘍における遺伝子異常の網羅的解析 | 平本 展大 | |
| 造血器疾患における遺伝子異常の網羅的解析研究 | 平本 展大 | |
| 初発節外性NK/T細胞リンパ腫における治療実態把握と予後因子解析を目的とした国際共同研究プロジェクト | 平本 展大 | |
| 劇症型再生不良性貧血に対する同種造血幹細胞移植 | 平本 展大 | |
| レセプトデータを用いた患者状態判定のためのアルゴリズム開発 | 下村 良充 | |
| 本邦における POEMS 症候群自家移植症例の長期予後の解析 | 平本 展大 | |
| 組織球症の標準治療確立を目的としたレジストリおよびバイオレポジトリの構築 | 近藤 忠一 | |
| エミシズマブによる出血予防使用下における高力価第VIII因子インヒビターを有する後天性血友病Aに対するリツキシマブの有効性と安全性に関する後方視的観察研究 | 西久保 雅司 | |
| 同種造血幹細胞移植後のAIH-like hepatitisの臨床的特徴と転帰に関する後方視的観察研究 | 近藤 忠一 | |
| CAR-T細胞療法抵抗性・耐性に関わる分子病理学的メカニズムの解明研究 | 永井 雄也 | PDF 別紙1 別紙2 別紙3 |
| 急性骨髄性白血病におけるFLT3-ITD変異の微小残存病変測定とその臨床的意義 | 近藤 忠一 | |
| 再発/難治性大細胞型B細胞リンパ腫に対するaxicabtageneアキシカブタゲン ciloleucelシロルユーセルの安全性と有効性 | 永井 雄也 | |
| 新規自動血球分析システムの分析性能の検証研究 | 近藤 忠一 | |
| 再発・難治性大細胞型B細胞リンパ腫に対するLisocabtagene maraleucel 治療の多施設共同観察研究 – JSCT CART23 – | 近藤 忠一 | PDF 別紙 |
| 血栓性血小板減少性紫斑病(TTP)に生じる心筋虚血と好中球細胞外トラップ(NETs)の評価 | 平本 展大 | |
| 血管免疫芽球性T細胞リンパ腫(AITL)とAITLに併発した血液腫瘍の遺伝学的検討 | 馬場 晟弥 | |
| びまん性大細胞型B細胞リンパ腫の患者における腫瘍細胞の表面抗原と予後の関連に関する後方視的観察研究 | 近藤 忠一 | |
| エミシズマブ承認前後の後天性血友病Aの臨床的特徴と転帰に関する後方視的観察研究 | 近藤 忠一 | |
| アゾール系抗真菌薬および分子標的薬のPK/PD評価 | 近藤 忠一 | |
| 再発難治性急性リンパ性白血病における治療予測のための腫瘍免疫プロファイリングの開発 | 近藤忠一 | |
| 血液疾患患者における新型コロナウイルス中和抗体薬の臨床データおよび治療経過に関する疫学観察研究 | 近藤忠一 | |
| アグレッシブATLにおける予後因子の検討と個別化医療の確立を目的とした全国一元化レジストリおよびバイオレポジトリの構築 | 平本展大 | |
| 血液疾患患者に関する診療データベースを用いた予後等の検討 | 下村良充/ 石川隆之 |
問い合わせ先
神戸市立医療センター中央市民病院 血液内科
(部長)近藤 忠一
住所:神戸市中央区港島南町2丁目1-1
電話番号:078-302-4321
お知らせ
医師、医師を目指す方向け当科のご紹介
※本コンテンツは、医師の方を対象とし、当医療機関についての理解を深めていただけるよう作成しているものであり、一般の方を対象とする宣伝・広告等を目的としたものではありません。
はじめまして、私は神戸市立医療センター中央市民病院血液内科部長の近藤 忠一(こんどう ただかず)と申します。
当科には8名の常勤医師と6名前後の専攻医が在籍し、造血器腫瘍患者(急性白血病、悪性リンパ腫、多発性骨髄腫、骨髄増殖性腫瘍、骨髄異形成症候群など)と非腫瘍性血液疾患患者(再生不良性貧血、特発性血小板減少性紫斑病など)の診療を行っています。年間の新規受診患者数は250-300名で、造血幹細胞移植も自家移植、同種移植合わせて年間50-60件ですが、患者数、移植件数ともに近畿圏で有数の規模です。新規薬剤の治験の依頼も多くいただいており、積極的に取り組んでいます。
近年、造血器腫瘍の治療に多くの分子標的薬剤が用いられるようになりました。特に悪性リンパ腫や急性リンパ性白血病に対してCAR-T療法という画期的な治療法が導入され、本邦でも2019年3月より保険承認されました。アフェレーシス産物の扱い (無菌調整など) や細胞製剤の凍結保存に関しては、厳密な細胞管理体制が求められるため、CAR-T治療は限られた施設でのみ認可されています。当院では早期導入を目指し、薬剤部・輸血検査管理室・細胞遺伝子検査室・臨床工学技術部と連携して運用体制を構築した結果、市中病院としてはかなり早く、2020年11月に施設認定されました。他院からCAR-T療法を希望される症例も積極的に当院で治療をお引き受けさせていただきます。
近藤 忠一
血液内科 部長
当科におけるリンパ系腫瘍に対する新規治療の取組
近年急性リンパ性白血病や悪性リンパ腫に対して画期的な治療法が開発されていることをご存知でしょうか。
従来これらの疾患に対しては、殺細胞性の抗ガン剤とリツキシマブなどの抗体製剤、さらには一部の急性リンパ性白血病に対してはイマチニブなどの分子標的薬も併用した治療を行ってきました。治療に難渋する場合には自家末梢血幹細胞移植を併用した大量化学療法や、同種造血幹細胞移植も行われてきました。
ニボルマブなどの免疫チェックポイント阻害剤の開発により、Tリンパ球は目覚ましい抗腫瘍効果を発揮しうることが明らかとなりました。残念ながら免疫チェックポイント阻害薬が有効な造血器腫瘍は限られています。しかし、患者さんのTリンパ球の力を使って血液がんを克服する治療法がBiTE、もしくはCAR-Tという新規技術を用いて開発され、従来の抗がん剤や分子標的薬剤に劣らない驚くべき治療効果が得られています。
現在のところはBiTEもCAR-Tも保険適応があるのはCD19抗原を発現する腫瘍に対してのみで、BiTEの対象となる疾患は前駆B細胞性急性リンパ性白血病のみ、CAR-Tも前駆B細胞性急性リンパ性白血病とびまん性大細胞B細胞性リンパ腫に限られます。BiTEは血液専門医の在籍する病院であればどの医療施設でも使用可能ですが、CAR-Tが行える施設は限定されます。2021年1月時点で公表されているのは全国で19施設、近畿圏で6施設、兵庫県では当院を含んで2施設のみです。
それではCAR-Tの詳細を当科の吉岡 聡医長より説明させていただきます。
再発又は難治性B細胞性急性リンパ芽球性白血病・びまん性大細胞型B細胞リンパ腫に対する新しい細胞治療:CAR-T細胞治療
はじめまして、神戸市立医療センター中央市民病院 血液内科医長の吉岡 聡(よしおか さとし)と申します。
本稿では、国内初のCAR-T細胞治療法として承認され、従来の治療では治癒が難しかった、難治性B-ALL、DLBCLの患者さんに対する新たな治療法として2019年5月には保険適応となったCD19を標的とするCAR-T細胞治療製剤: tisagenlecleucel(キムリア, ノバルティスファーマ株式会社)について紹介致します。
当時、1回3,349万円という治療費用が世間でも話題となり名前だけは聞いたことがあるという方も多いかと思いますが、キムリア治療実施の意義について知っていただくきっかけになれば幸いです。
- 「キムリア」はノバルティスファーマ株式会社の登録商標です。
血液内科 医長
吉岡 聡(よしおか さとし)
専門分野
血液疾患全般
学会専門医・認定医
日本内科学会認定医・総合内科専門医・指導医
日本血液学会認定血液専門医・指導医
日本造血細胞移植学会認定医 など
キムリア治療の概要
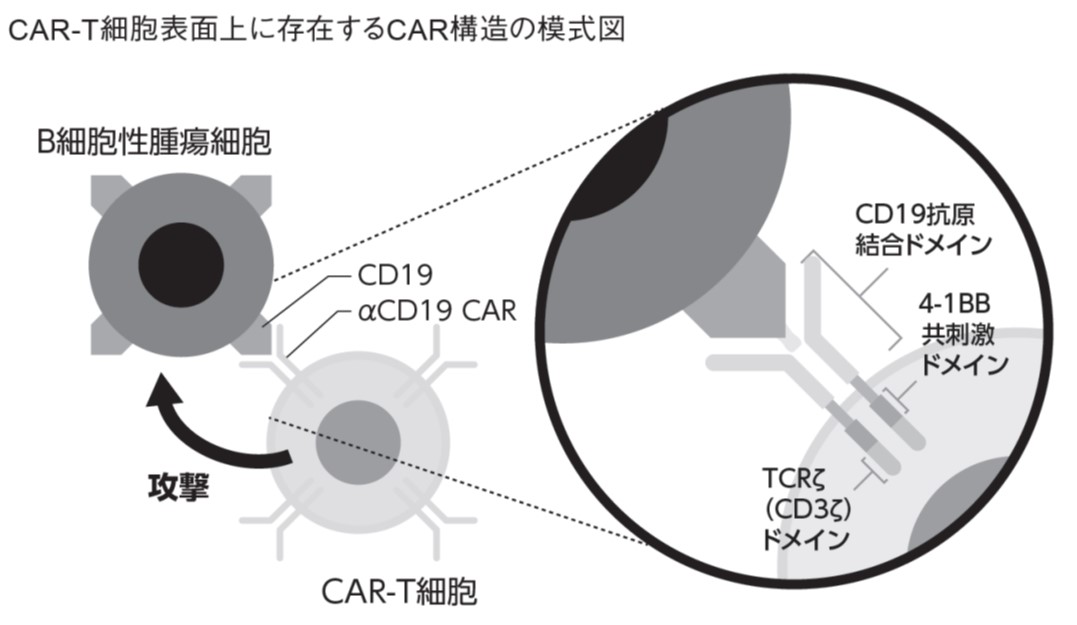
キムリアはB細胞腫瘍の表面に発現するCD19抗原をターゲットに作用するキメラ抗原抗体(Chimeric Antigen Receptor: CAR)をあらかじめ採取した患者T細胞に遺伝子導入して得られるCAR-T細胞による細胞治療製剤です。
CAR-T細胞は腫瘍細胞と反応すると活性化され、perforinやgranzymeなどの細胞障害蛋白を放出し、腫瘍細胞を細胞死に誘導することにより抗腫瘍効果を発揮します。腫瘍細胞に対するドナーT細胞による抗腫瘍効果に期待する同種造血幹細胞移植とは異なり、自己T細胞を使用することにより、同種移植でみられるような移植片対宿主病(GVHD)やその治療による易感染性などの有害事象を避けることができる点で優れています。
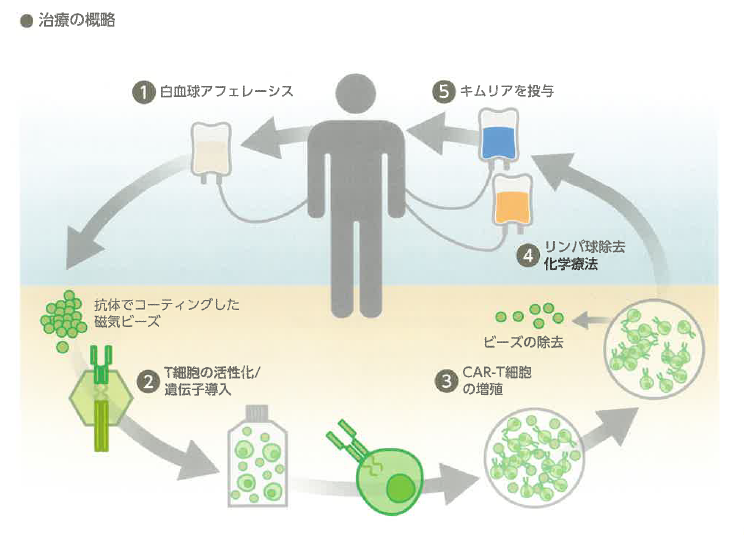
キムリア治療は患者T細胞を採取することから始まります(白血球アフェレーシス)。すなわち、キムリアを製造する過程の一部を医療機関が担う点がこれまでの治療とは大きく異なります。
もちろん製剤には一定の基準を満たす品質が求められるため、実施医療機関として認定されるためには厳格な審査をクリアする必要があり、全国でも本治療の提供可能施設はまだまだ限られています。当院では、その審査による認定を得て、2020年12月から実施可能となりました。
キムリアの対象となる疾患及び病状
本邦では、再発又は難治性のCD19陽性のB細胞性急性リンパ芽球性白血病(以下、B-ALL)、再発又は難治性のCD19陽性のびまん性大細胞型B細胞リンパ腫(以下、DLBCL)に対する効能で保険適応となっています。高額かつ実施医療機関が限られるため、両疾患ともキムリア治療実施には慎重な適応の判断が求められます。
両疾患に共通の適応除外要件は、下記のとおりです。
- CD19抗原陽性が確認されない患者
- 中枢神経病変がある場合
- 他の悪性腫瘍を合併している患者
- キムリア投与歴のある患者(再治療は認められていない)
B-ALLには投与時に25歳以下と年齢制限が設けられています。
なお、B型又はC型肝炎ウイルスキャリア、既往患者は慎重投与(活動性感染のある患者、HBc抗体単独陽性の患者は適応外)、HIV感染患者は適応外となっています。当然ながら、活動性感染のある患者、重度の臓器機能障害のある患者も適応外となります。
以下に疾患別の適応について具体的に詳述致します。ただ、実際は患者さんの病状や既往症はさまざまですので、適応がありそうだと判断された時点で、気軽にご相談ください。
1. 再発又は難治性のCD19陽性B-ALL
1)適応が考慮される患者
:以下のいずれかの条件を満たし、造血幹細胞の採取ができない、合併症がある等の理由で自家造血幹細胞移植の適応とならない、または自家造血幹細胞移植後に再発した患者。
- 初発難治性DLBCL:化学療法を2ライン以上施行し、完全奏効が得られなかった又は完全奏効が得られたが再発した患者。
- 再発難治性DLBCL:再発後に救援化学療法を1ライン以上施行し、完全奏効が得られなかった又は完全奏効が得られたが再発した患者。
- 濾胞性リンパ腫から形質転換したDLBCL:通算2ライン以上の化学療法を施行し、かつ形質転換後に少なくとも1ラインの化学療法を施行し、完全奏効が得られなかった又は完全奏効が得られたが再発した患者。
2)適応とならない患者
- 同種造血幹細胞移植の治療歴のある患者
- リツキシマブ及びアントラサイクリン系抗悪性腫瘍剤を含む化学療法歴のない患者
- T-cell/histiocyte-rich large B-cell lymphoma(THRBCL)、皮膚原発DLBCL、縦隔原発B細胞リンパ腫(PMBCL)、EBV陽性DLBCL(高齢者)、リヒター症候群及び、バーキットリンパ腫を有する患者
キムリアの治療成績
キムリア治療の適応は前述のように、従来の治療では治癒が難しい患者が対象となります。実際、従来であれば治療手段がなく緩和ケアに移行していた患者さんがキムリア投与を受けられ、治療後半年も経たず職場復帰されたというケースもあります。
ここでは具体的な治療成績について、臨床試験の結果、最新の論文報告から紹介します。
1. 再発又は難治性のCD19陽性B-ALL
はじめに保険収載の根拠になった国際共同第II相試験(ELIANA試験)の結果を紹介します。
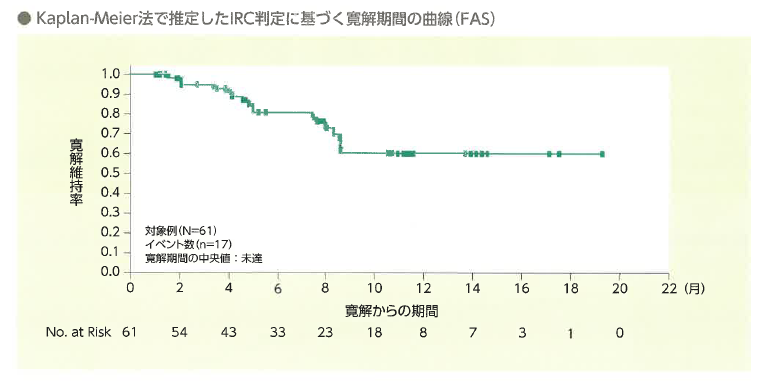
年齢中央値11(3-23歳)の再発・難治性B-ALL患者75例に投与がおこなわれました。追跡期間中央値は投与から9.92(0.4-22.8)か月、血液学的完全寛解(hCR)を61例(82%)に認め、12か月時点での寛解維持率は59%でした。(N Engl J Med. 2018;378:440)
実臨床における治療成績としては、2020年に米国国際血液移植血液センター(CIBMTR)のレジストリデータの解析が報告されました。(Blood Adv. 2020;4:5414-5424)
年齢中央値13.2(0.4-26.2)歳の再発・難治性B-ALL255例に投与され、追跡期間中央値13.4(3.5-27.9)か月、hCR率は85.5%、12か月寛解維持率は60.9%とELIANA試験と同様の結果が得られています。
2. 再発又は難治性のCD19陽性DLBCL
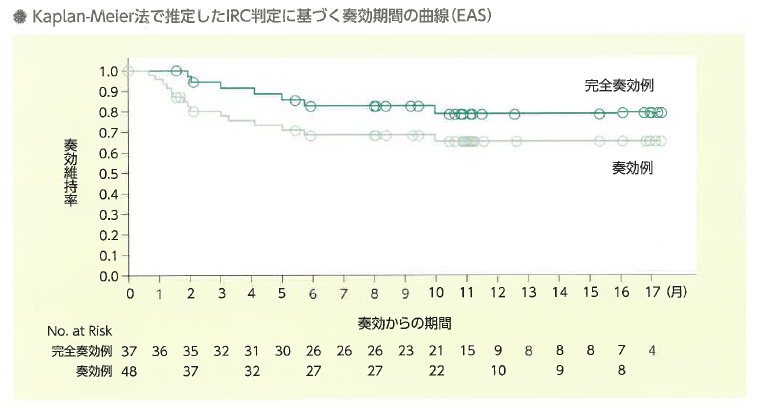
次に再発・難治性DLBCLを対象とした国際共同第II相試験(JULIET試験)の結果を紹介します。年齢中央値56(22-76)歳の再発・難治性B-ALL患者111例に投与がおこなわれました。追跡期間中央値は投与から14(0.1-26)か月、評価可能93例のうち完全寛解(CR)37例(40%)を含む奏効率(ORR)は52%、12か月奏功維持率はは65%でした。(N Engl J Med. 2019;380:45)
実臨床における治療成績はB-ALL同様CIBMTRのレジストリデータの解析の報告では、年齢中央値65(18-88歳)の155例に投与され、CR率は40%、ORRは62%、奏効例の12か月無増悪生存率は48.4%とJULIET試験よりやや劣る結果でした。より高齢の患者に投与されていることが影響しているのかもしれません。
3. キムリア治療の有害事象
キムリア治療特有の有害事象としてサイトカイン放出症候群(CRS)、免疫エフェクター細胞関連神経毒性(ICANS)が知られています。
CRSは増殖・活性化したCAR-T細胞が産生するサイトカインによって引き起こされる全身性炎症反応です。臨床試験では投与から発症までの期間の中央値は3日で持続期間の中央値は約1週間と報告されています。重症化した場合、人工換気、高用量の昇圧剤などICUでの管理を要する場合もあります。
ICANSの発症機序は未解明ですが、発症までの期間の中央値は7日とCRSと異なるため、別の病態と考えられています。多くは一過性であまり治療を要さず回復します。
これらに備え、当院では、キムリア投与前からICU医師、脳神経内科医師と連携を取り有事に即時の対応ができる体制を整えています。 長期的には低ガンマグロブリン血症による易感染性があり、ガンマグロブリンの補充が必要になります。
また、有害事象ではありませんが、製造がうまくいかないケースが約1割存在するという問題もあります。
難治性B-ALL、DLBCL患者がいらっしゃればご紹介ください
キムリア治療は再発・難治性B-ALL、DLBCL患者さんにとって大きな福音となる治療です。高額な治療であること、長期の治療成績の情報が不十分であることなど克服していくべき問題もありますが、他のCD19陽性B細胞腫瘍はもちろんのこと、標的抗原を変えることによって他の難治性造血器腫瘍や固形癌の治療にも応用が広がることが期待されます。 当院は隣接する公益財団法人神戸医療産業都市推進機構が本邦初のキムリア製造・供給施設になるという立地のメリットも生かして、難治性B-ALL、DLBCL治療に貢献していく所存です。25歳以下の難治性B-ALL患者さんや、臓器機能が保たれた難治性DLBCL患者さんがおられましたら、キムリア治療の適応を確認したいという問い合わせの段階でも構いませんので、遠慮なく地域連携室を通してご紹介いただけますと幸いです。もちろん、造血幹細胞移植を始め、あらゆる血液疾患の診療についても広くご紹介をお待ちしています。