ごあいさつ
神戸市立医療センター中央市民病院は、「神戸市の基幹医療施設として、市民の生命と健康を守るため、患者中心の質の高い医療を安全に提供する」ことを基本理念とし、24時間体制での救急医療の実践、高度先進医療の提供、などを基本方針として掲げています。一方で、当院ではより安全でより有効な医療を開発するために、以前から治験や臨床研究に取り組んできました。2017年11月には隣接する先端医療センター病院を統合したことを機に臨床研究推進センター(Center for Clinical Research and Innovation)を立ち上げ、現在に至っております。治験や臨床研究を行うためには、様々な法令、規則が定められています。それらを正しく理解し、被験者の保護、データの信頼性や倫理性・科学性の確保のための手順、資金や支援の透明化などが厳格に求められております。当センターでは患者さんの権利や安全を守り、新しい医療の開発に積極的に貢献するため、今後も治験や臨床研究を管理、支援する組織と機能の拡充に努めていきたいと考えています。みなさまのご支援ご協力をお願い申し上げます。
臨床研究推進センター長 橋田 亨
理念と事業
理念
- 臨床研究を積極的に推進することにより、先端医療をいち早く提供し市民の健康の増進に貢献するとともに、生命の維持と生活の質の向上につながる新たな医療を創造し、神戸医療産業都市の発展に寄与する。
- 医薬品医療機器等の治験を含む臨床研究を適切に実施するため、法令や指針に則り、倫理性と科学性を確保するとともに、円滑かつ安全に研究を遂行できるよう、研究を管理し、研究者と被験者を支援する。
事業
- 医薬品医療機器等の企業治験、医師主導治験の推進と管理
- 特定臨床研究を主体に医師主導臨床研究の推進と管理
- 研究者、依頼者の相談と支援業務
- 被験者の保護と支援業務
- 臨床研究推進に関する教育
- 他の医療機関の臨床研究の推進に関する業務
- その他必要な事業
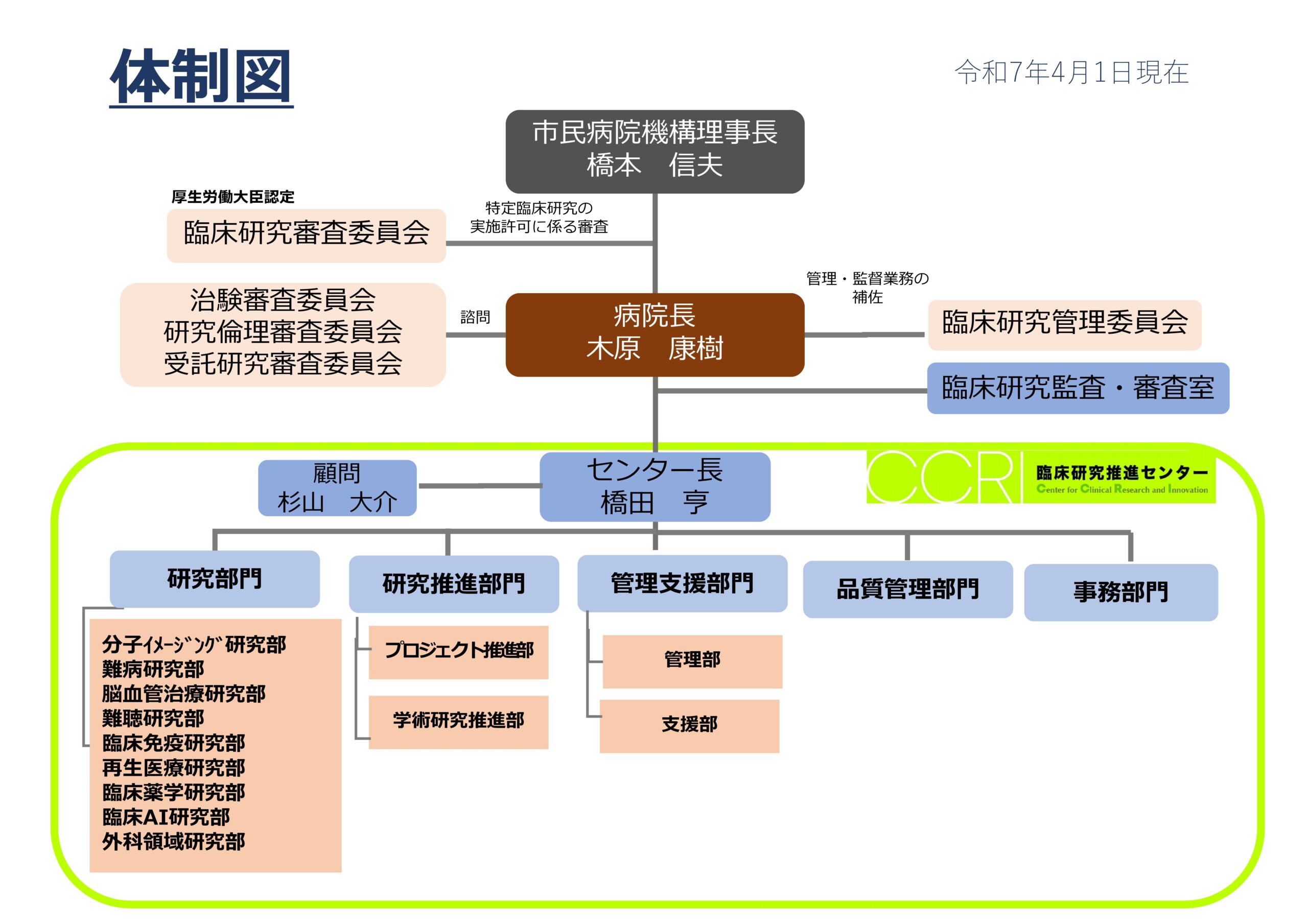
組織図
活動方針・活動内容
研究部門
分子イメージング研究部
分子イメージングとは、生体内で行われている細胞レベル・分子レベルの活動を画像化することです。分子イメージング研究部は、生体機能を画像化する「PET」という診断技術を活用し、疾患の病態解明や新薬開発に取り組んでいます。
当研究部の主なミッションとして、PETに関する治験薬製造と治験実施、臨床研究、撮像・解析法開発と品質管理があります。また市民病院として、臨床研究を通じて得られた最先端の研究成果を市民の皆様に提供する体制づくりに努めております。これまでに培った創薬・撮像・品質管理のノウハウを最大限に活用し、PETを用いた安全かつ有効性の高い診断技術を開発し、医療の進歩に貢献していきます。
難病研究部
難病研究部では、「アクテリオン ファーマシューティカルズ ジャパン株式会社」(現ヤンセンファーマ株式会社)からの寄付金を原資に、遺伝子異常の存在が疑われる患者を対象にゲノム解析や遺伝子発現解析を行い診断を確定するとともに、これらの情報を匿名化した上で発症機構の解明に取り組んだほか、パーキンソン病や筋萎縮性側索硬化症の指定難病や急性骨髄性白血病、口腔癌、貧血合併心不全といった難治性疾患に対する新しい治療法や診断法等の開発に向けた研究にも取り組んでいます。
脳血管治療研究部
脳血管治療に関する治験や多施設共同研究のマネジメント及び脳血管治療関連医療機器の開発(機器開発研究)を研究者や企業と共同で積極的に進めています。
難聴研究部
人工内耳術後の①視覚野の発達(音声刺激に対する反応)と、②視聴覚統合(聴覚野と視覚野の同期的活動)に注目し、人工内耳装用児でも施行可能な光トポグラフィー(fNIRS)と多チャンネル脳波計を用いた脳機能評価を行う臨床研究を実施し、新たなリハビリテーションプログラムの開発につなげていきます。
臨床免疫研究部
神戸医療産業都市推進機構・先端医療研究センター免疫機構研究部で研究が行われている「抗PD-1アゴニスト抗体による治療法」や「炎症性疾患の早期診断マーカー」の臨床開発を推進するため、同研究部と連携して、関連する臨床基盤の構築や臨床研究の企画・実施・管理を行うとともに、免疫疾患等に関わるエビデンスの構築と、新規治療法・診断法開発へのフィードバックを図ります。
再生医療研究部
幹細胞を用いた中枢神経疾患に対する細胞移植治療の研究を行うとともに移植後免疫反応の制御の研究などに取り組んでいます。まず専門である神経領域においてパーキンソン病の再生医療の臨床応用を進めながら、将来には他領域での再生医療の展開を図り、再生医療を当院の強みの1つとして発展を目指していきます。
詳しくはこちら臨床薬学研究部
臨床薬学研究部では医薬品の適正使用に関わる様々なテーマについて臨床薬学研究により解決方法を見出し、実臨床の場に提案・実装してその効果を検証します。そして医師が実施する臨床研究において、体内動態・薬力学(PK/PD )プロトコルの提案、薬物濃度測定、薬物動態解析などにより、臨床薬理学的支援を行います。
また、神戸学院大学大学院薬学研究科との間で締結している「教育・研究協力に関する協定」に基づき、研究者の育成にも取り組んでまいります。
臨床AI研究部
臨床AI研究部は、人工知能(AI)を活用し、より質の高い医療の提供を目的とした臨床研究を行っています。これに並行して、医療の質と安全性の向上、さらには業務の効率化を図るため、日常の診療活動においてもAIの活用を推進しています。兵庫県立大学と密接に連携することにより、診療データを安全かつ低コストで分析することができる体制を整えています。
現在実施中の研究
| 研究課題名 | 説明文(PDF) |
| 離散事象型シミュレーションモデルを用いた看護業務量予測に関する研究:後向き観察研究 | |
| 人工知能(AI)を用いた退院サマリーの質評価に関する研究:後向き観察研究 | |
| 患者見守りカメラ映像を用いたマルチモーダルAIによるケアプロセス解析:前向き観察研究 |
外科領域研究部
当研究部は、「手術を科学に」をモットーとしてロボット手術を中心とした低侵襲手術がもたらす臨床成果をもとに、新しい診断・治療法や医療機器・システム開発につながる研究体制の構築を目指しています。さらに、手術で得られる切除検体から保存可能なバイオマテリアルを作製し、そこに当院の高度医療機能が産み出す様々な診療情報を統合することで、“医療情報を纏った生きた研究試料”としてその価値を高めて、メディカルクラスターへ提供します。
このように、“手術”と“研究”が相乗的に発展する仕組みとして、“Patient-Derived Material for Medical Innovation Hub(PD-MiH)”を機能させ、様々な研究分野へ貢献することを目指します。
研究推進部門
研究推進部門は、当院における多様な研究活動を専門的かつ実践的に支援する組織です。「プロジェクト推進部」と「学術研究推進部」で構成され、医療機器・医薬品等の開発支援から、院内の学術活動の推進まで幅広く対応しています。いずれの部門も臨床現場を起点とした課題や疑問の解決を使命としており、当院の高い臨床力を社会に発信するため、質の高い研究活動の推進と成果の創出に努めてまいります。
プロジェクト推進部
臨床の課題を研究テーマへと繋げるために臨床現場観察を導入し、医療機器や医薬品等の開発を推進するとともに、医師主導治験の調整事務局や多施設共同研究の主任研究者・研究事務局を当院医師が担当する場合に、その計画や運用を専門的に支援します。
また、データ集積管理システムであるREDCapを活用したデータ管理にも注力し、臨床研究等のプロジェクトを推進しています。
学術研究推進部
学術研究に関わる院内の様々な取り組み(研究相談、統計解析相談、データ入力、イラスト作成、英文校閲など)を支援しています。
管理支援部門
管理支援部
管理支援部は、倫理的かつ科学的に質の高い臨床試験(治験)を安全に円滑に実施するため、臨床研究に関する専門的知識をもつスタッフによる管理部門と臨床研究コーディネーター(CRC)を中心とした支援部門から構成されます。高度な専門性を駆使して、患者さんの権利や安全を守りつつ、質の高い医療の提供に貢献していきたいと考えています。
品質管理部門
品質管理部
品質管理部は、臨床研究の質を確保することを目的とした部門です。院内の様々な部門と協働し、各種法令指針に基づいて研究者が実施した臨床研究を病院として適正に管理することで、臨床研究に参加された患者さんの保護や信頼性のある臨床研究の遂行に努めていきます。
事務部門
事務部
事務部門では、臨床研究・治験に係る契約、文書管理などの事務全般、安全情報の集約、知財管理、収支管理、広報・企画調整に関する業務等及び「KOBEもの忘れネットワーク事務局」を担っています。また、臨床研究推進センター内の事務を統括するとともに他部門と密接な連携を図り、臨床研究・治験の円滑な実施を支えるために事務的サポートをしています。
KOBEもの忘れネットワーク 公式ウェブサイトはこちら活動状況・実績
治験実績(年度別)
| 年度 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|---|
| 新規企業治験 | 35 | 30 | 31 | 42 | 36 | 25 |
| 新規医師主導治験 | 3 | 3 | 6 | 5 | 3 | 4 |
| 延べ実施数 | 173 | 167 | 169 | 180 | 180 | 183 |
主な実施診療科・対象疾患
| 循環器内科 | 慢性心不全、心房細動、末梢動脈疾患など |
|---|---|
| 糖尿病・内分泌内科 | 糖尿病性腎臓病、糖尿病性神経障害 |
| 腎臓内科 | 腎性貧血、急性腎傷害 |
| 脳神経内科 | パーキンソン病、POEMS症候群、ギラン・バレー症候群、筋萎縮性側索硬化症など |
| 消化器内科 | B型慢性肝炎、C型慢性肝炎、十二指腸潰瘍、逆流性食道炎、クローン病など |
| 呼吸器内科 | 肺癌、喘息、慢性閉塞性肺疾患(COPD)、特発性肺線維症など |
| 血液内科 | 悪性リンパ腫、多発性骨髄腫、白血病、骨髄異形成症候群、骨髄線維症、移植片対宿主病(GVHD)など |
| 腫瘍内科 | 胃癌、大腸癌、食道癌、膵癌、頭頸部癌、原発不明癌、肺癌、抗がん剤による支持療法など |
| 緩和ケア内科 | 癌性疼痛 |
| 精神・神経科 | アルツハイマー型認知症 |
| 小児科 | 小児気管支喘息 |
| 皮膚科 | アトピー性皮膚炎、帯状疱疹後神経痛 |
| 外科・移植外科 | 外科術野消毒 |
| 乳腺外科 | 乳癌 |
| 脳神経外科 | 脳卒中、脳動脈瘤、頸動脈狭窄症など |
| 整形外科 | 変形性関節症、難治性骨折 |
| 産婦人科 | 子宮頸癌、子宮筋腫 |
| 泌尿器科 | 前立腺癌、膀胱癌、腎細胞癌など |
| 耳鼻咽喉科 | 難聴 |
| 麻酔科 | 術後鎮静 |
| 放射線診断科 | アルツハイマー型認知症(診断)、PET検査受託試験 |
| 救急部 | 敗血症に伴う頻脈性不整脈 |
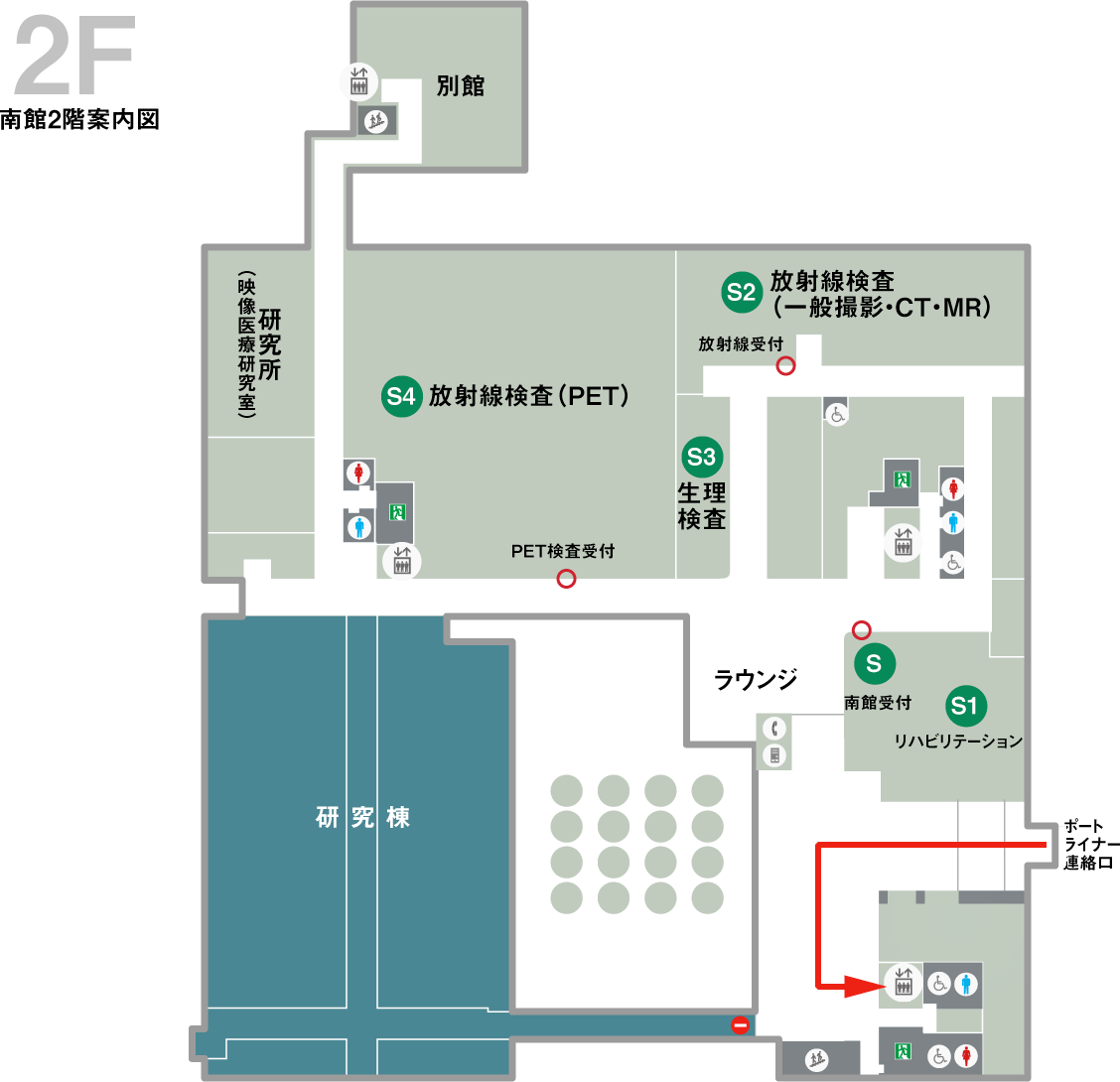
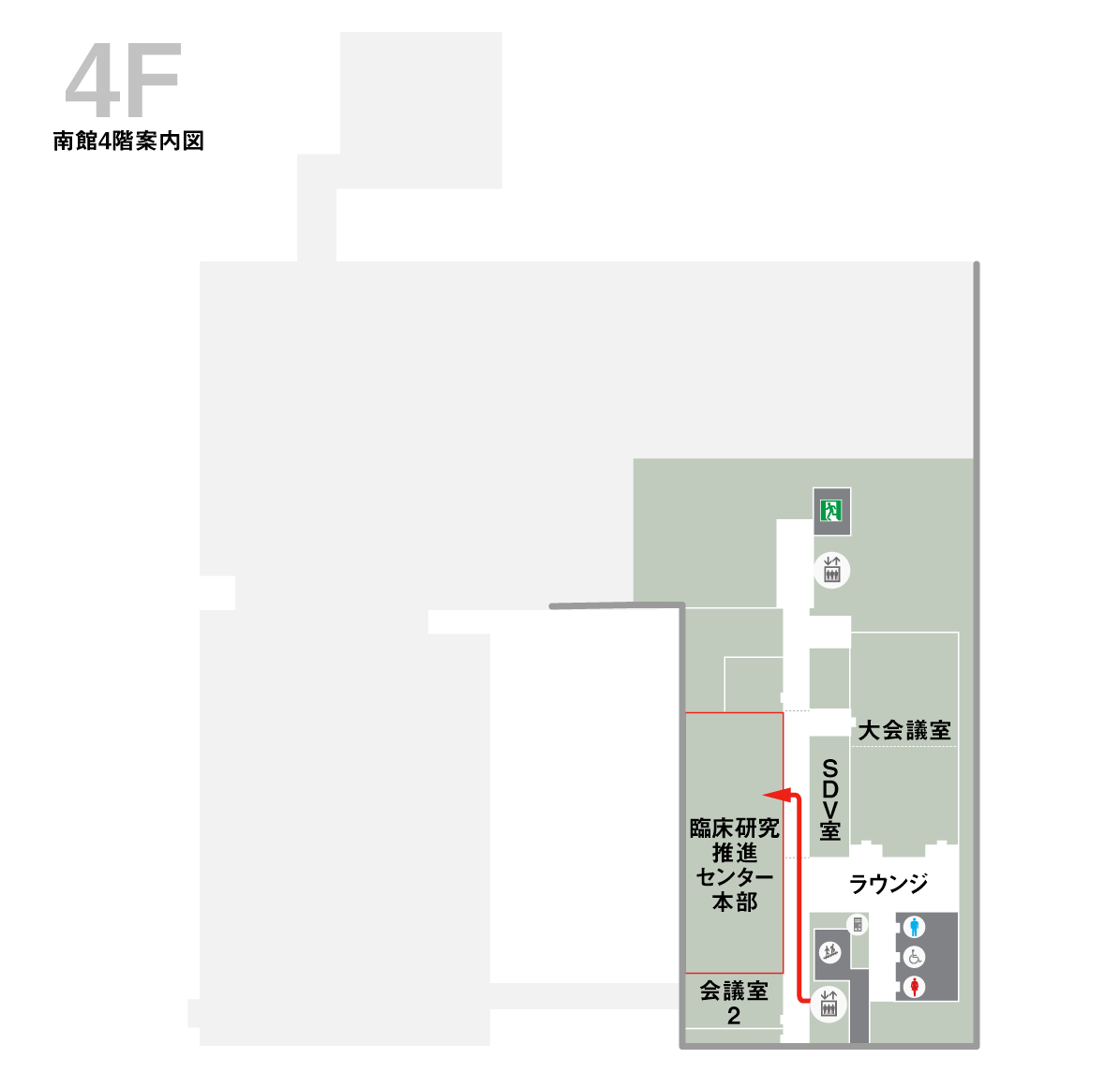
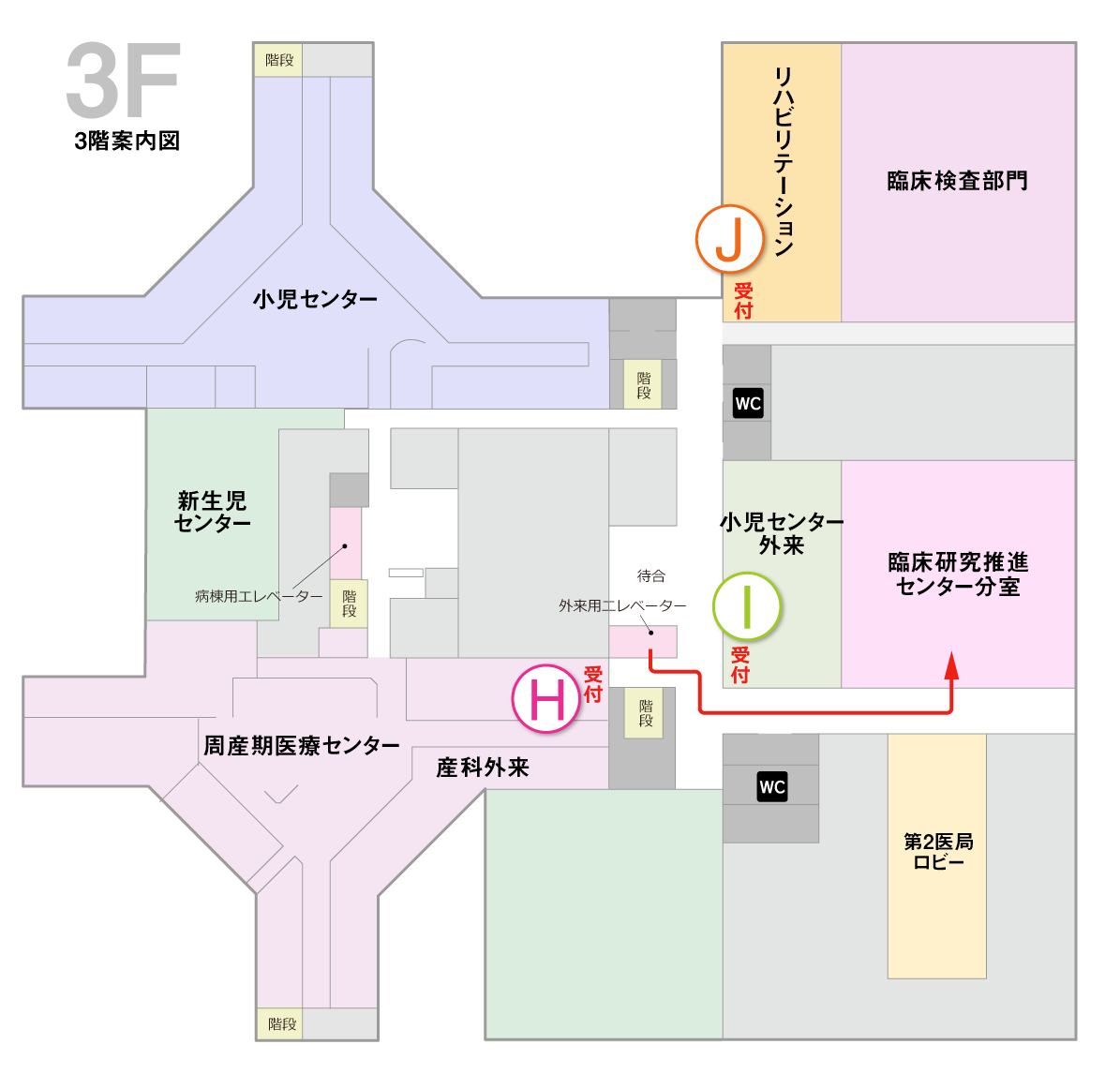
センターへのアクセス
臨床研修推進センターは中央市民病院の本館と南館の2箇所にございます。
当院の臨床研究コーディネーター(CRC)にご用の方は、下記の分室へお越しください。
病院への交通アクセスはこちらをご参照ください。臨床研究推進センター本部(南館4階)
〒650-0047
神戸市中央区港島南町2丁目2番地
ポートライナー連絡口を入って左側のエレベータで4階までお越しください。
臨床研究推進センター分室(本館3階)
〒650-0047
神戸市中央区港島南町2丁目1番地1
問い合わせ
治験・臨床研究に関する患者相談窓口
〒650-0047
神戸市中央区港島南町2丁目1番地1
臨床研究推進センター
TEL:(078)302-4448 ※電話応対は平日9:00〜17:00
FAX:(078)302-4604
メール:c_ccri1あっとkcho.jp
※迷惑メール対策のため、メールアドレスの あっと は@に置き換えてください。
治験依頼者からの治験に関する連絡先
TEL:078-302-4499 ※電話応対は平日9:00~17:00
Mail:c_ccriあっとkcho.jp
※迷惑メール対策のため、メールアドレスの あっと は@に置き換えてください。
研究者・企業からの臨床研究に関する連絡先
TEL:078-302-4499 ※電話応対は平日9:00~17:00
Mail:rinkenあっとkcho.jp
※迷惑メール対策のため、メールアドレスの あっと は@に置き換えてください。
研究者・企業からの特定臨床研究に関する連絡先
TEL:078-302-4499 ※電話応対は平日9:00~17:00
Mail:c_tokuteiあっとkcho.jp
※迷惑メール対策のため、メールアドレスの あっと は@に置き換えてください。
製造販売後調査全般の窓口
TEL:078-302-4499 ※電話応対は平日9:00~17:00
Mail:chikenあっとkcho.jp
※迷惑メール対策のため、メールアドレスの あっと は@に置き換えてください。