部長あいさつ
私たち薬剤師は、責任をもって安全で効果的な薬物治療の提供に貢献しています。調剤室の中はもとより、薬剤師外来、入院前準備センター、救命救急センター、化学療法センター、入院中の病棟薬剤業務、手術室での薬剤業務などの薬物治療のあらゆる場面で、患者さんへの服薬指導から副作用のモニタリングまで継続した薬学的管理を実践しています。そして、一人一人の患者さんの日常生活を考え、退院後も患者さんが安心して薬物治療を続けられるように、保険薬局とも連携を深め、患者さんの健康をサポートしていきます。さらに、投与後の体内動態・反応性、副作用対策に関する研究により薬のプロファイルを科学し、薬の治療効果を高め、副作用を減らす取り組みを進めて安全な薬を育てていきます。
『薬あるところに薬剤師あり』を念頭に置き、患者さまに安心して、満足していただけるように、薬剤部員一丸となって取り組んでまいります。お薬に関してご質問・ご相談がある方は、お気軽に薬剤師にお声かけ下さい。
令和6年 4月
薬剤部スタッフ
令和7年 4月
| 薬剤部部長 | 室井 延之 |
|---|---|
| 薬剤部副部長 | 久米 学 |
| 薬剤部副部長 | 尾山 将樹 |
| 薬剤部主幹 | 小田 稔彦 |
| 薬剤部主幹 | 大音 三枝子 |
| 院長補佐 兼 参事 | 橋田 亨 |
ほか主査11名、主任4名
常勤薬剤師 計73名、非常勤薬剤師4名、薬剤師レジデント9名
| 薬剤師 | 73(常勤)、4名(非常勤) |
|---|---|
| 薬剤師レジデント | 9名(定数10名) |
| 事務員・補助業務 | 10名 |
| 合計 | 96名(薬剤師86名) |
指導・専門・認定薬剤師
令和7年 4月
| 日本医療薬学会 | 医療薬学指導薬剤師 | 4名 |
|---|---|---|
| 医療薬学専門薬剤師 | 7名 | |
| がん指導薬剤師 | 1名 | |
| がん専門薬剤師 | 5名 | |
| 薬物療法専門薬剤師 | 2名 | |
| 日本病院薬剤師会 | がん薬物療法認定薬剤師 | 3名 |
| HIV感染症認定薬剤師 | 1名 | |
| 感染制御認定薬剤師 | 2名 | |
| 日病薬病院薬学認定薬剤師 | 32名 | |
| 生涯研修履修認定薬剤師 | 10名 | |
| 日本臨床腫瘍薬学会 | 外来がん治療認定薬剤師 | 4名 |
| 糖尿病療養指導士認定機構 | 糖尿病療養指導士 | 3名 |
| 日本くすりと糖尿病学会 | 糖尿病薬物療法認定薬剤師 | 1名 |
| 日本医療情報学会 | 医薬品情報認定薬剤師 | 1名 |
| 医療情報技師 | 1名 | |
| 日本緩和医療薬学会 | 緩和薬物療法認定薬剤師 | 4名 |
| 緩和医療暫定指導薬剤師 | 3名 | |
| 麻薬教育認定薬剤師 | 1名 | |
| 日本臨床栄養代謝学会 | NST専門療法士 | 5名 |
| 周術期・救急集中治療専門療法士 | 1名 | |
| 日本臨床救急医学会 | 救急専門薬剤師 | 1名 |
| 救急認定薬剤師 | 2名 | |
| 日本老年学会 | 老年薬学認定薬剤師 | 1名 |
| 日本中毒学会 | 認定クリニカル・トキシコロジスト | 1名 |
| 日本臨床薬理学会 | 認定CRC | 2名 |
| 日本化学療法学会 | 抗菌化学療法認定薬剤師 | 4名 |
| 日本循環器学会 | 心不全療養指導士 | 2名 |
| 日本腎臓病協会 | 腎不全療養指導士 | 1名 |
| 日本薬剤師研修センター | 研修認定薬剤師 | 4名 |
| 認定実務実習指導薬剤師 | 8名 | |
| 小児薬物療法認定薬剤師 | 2名 | |
| 漢方薬・生薬認定薬剤師 | 2名 | |
| 日本臨床栄養協会認定 | NR・サプリメントアドバイザー | 1名 |
| 兵庫県健康福祉部健康局疾病対策課 | 肝炎医療コーディネーター | 1名 |
| 糖尿病療養指導士兵庫県連合会 | 糖尿病療養指導士兵庫 | 1名 |
| 日本麻酔科学会 | 周術期管理チーム薬剤師 | 1名 |
| 日本リウマチ財団 | リウマチ財団登録薬剤師 | 1名 |
| 日本パーキンソン病・運動障害疾患学会 | パーキンソン病療養指導士 | 2名 |
薬剤部の業務について
処方箋調剤
入院および外来ともに電子カルテシステムと連携した調剤システム(調剤監査システム、自動薬袋作成、自動錠剤分包、処方監査システム)を稼働させることで、正確な調剤を行うとともに調剤の効率化を図っています。また、処方薬搬送システムの導入により、迅速かつ正確な薬剤の搬送が可能となっています。2021年には、ロボット調剤室(自動薬剤ピッキング装置、散薬調剤ロボット、ピッキングサポートシステム)を導入し、調剤のデジタル化を進めています。
注射薬調剤
注射薬オーダリングシステムと連動した自動注射薬払出装置を用いて、集中治療室を除く全ての病棟で、患者さんごと・施用ごとに1日単位でトレーに入れて払い出しています。患者さんの状態や投与量、投与方法、配合変化や副作用などをチェックし、安全な注射薬の提供に努めています。また、正確な調剤の実現するために、2023年には注射のピッキングサポートシステムを導入しました。
注射薬無菌調製
外来・入院全てのがん化学療法について処方内容のチェック後に安全キャビネット内で無菌調製を実施しています。2017年より更なる効率化を図るため、抗がん薬調製ロボットを導入しました。また、無菌的に混合する必要がある高カロリー輸液はクリーンベンチ内で調製しています。
製剤
製薬企業が販売する医療用医薬品では患者さんの病態に応じた多種多様なニーズに対応できない場合に、院内製剤を調製しています。院内製剤は、特定の患者さんにとって治療上不可欠な製剤であっても採算が取れない等の理由により製薬企業から販売されていないものを対象とし、医師の要望により病院内で審査したうえで調製しています。
薬剤管理指導業務・病棟薬剤業務
救命救急センターや集中治療室を含むすべての病棟に薬剤師を配置しています。患者さんのベッドサイドで服薬指導を行うとともに、患者さんのアドヒアランスの向上、自己管理への支援や副作用回避のための処方提案など、安全で効果的な薬物治療の提供に努めています。さらに、各種啓発教室(患者教室)等を通して地域医療にも積極的に参画しています。
患者総合支援部門
入院前準備センター業務
入院前に常用薬や健康食品、サプリメントの使用を確認して手術前中止薬を把握するなど、入院後の治療の円滑化を図っています。他の医療機関で処方された薬についても確認し、重複や相互作用をチェックします。さらに、手術を受ける患者さんの早期回復と社会復帰を目指して2021年に開設された周術期サポートセンターに薬剤師も参画しています。
退院支援業務
地域医療連携センターの薬剤師と病棟担当薬剤師が連携しながら退院支援カンファレンスや退院時共同指導(退院前カンファレンス)に参加し、退院後も患者さんが安心して薬の治療を続けられるように、患者さんの暮らしに合った在宅での服薬を支援しています。また、転院先医療機関への施設間薬剤情報提供書の作成や医療機関および保険調剤薬局等との連携により、退院後も安全で効果的な薬物療法を受けていただけるように努めています。
薬剤師連携業務
病院薬剤師と保険薬局薬剤師が連携・協力し、個別化対応による服薬指導を実施することでアドヒアランス確保に努めています。心不全患者の病状悪化による再入院の原因のひとつにアドヒアランス低下が挙げられており、その対応として2023年より心不全患者に対してトレーシングレポートを用いた保険薬局との薬剤師連携を開始しています。
薬物血中濃度モニタリング(TDM)

医薬品管理
医薬品の購入、保管および各部署への供給等を在庫管理システムで効率的に管理しています。各部署の医薬品等は薬剤師が定期的にチェックを行い、適切な数量・期限などの薬品管理に努めています。
麻薬管理
優れた鎮痛作用を持ちながらも厳密な管理を要する医薬品である医療用麻薬は、その取扱いと保管方法が厳しく規制されており、法に基づいて院内の医療用麻薬の管理及び適正使用を推進しています。また、手術室すべてにリアルタイム薬品管理装置(リテラ)を設置し、24時間の薬品管理を可能としています。さらに、手術室サテライトファーマシーに常駐している薬剤師と病棟担当薬剤師が連携し薬学的管理を行い、質の高い周術期医療に貢献しています。
医薬品情報管理業務

治験・臨床研究支援業務
臨床研究推進センターでは、新たな治療方法、医薬品・医療機器の開発や既存の医療技術の最適化を推進しています。薬剤師は管理・支援部門の中核として、倫理的かつ科学的に質の高い治験・臨床研究を安全で円滑に行うための支援業務を行っています。また、治験薬の適正な管理に加え、安全性に関する情報やその他の情報の収集・管理を行っています。
薬剤師外来

内服薬確認外来
専門ブースや入院前準備センターで入院前の患者さんの常用薬を調べ、当院採用の有無や代替薬の紹介、手術前中止薬の中止状況を確認します。
経口抗がん薬 薬剤師外来・間質性肺炎 薬剤師外来
医師の診察前に、抗がん薬・間質性肺炎治療薬の服薬状況や副作用について確認し、患者さんの状態をカルテに記載するとともに、必要に応じて医師に処方提案します。
C型肝炎治療薬 薬剤師外来
C型肝炎ウイルスに対する治療薬である直接作用型抗ウイルス薬の効果を最大とするために常用薬との相互作用を最小限にし、必要な服薬方法の指導および服薬状況の確認を行います。また、治癒後にも肝硬変・肝発がんリスクは残ることを説明し受診・受検継続の必要性についても説明しています。
サリドマイド・レナリドミド・ポマリドミド 薬剤師外来

HIV治療薬薬剤師外来
HIV治療薬の副作用チェックや病気の悩みなど、面談を随時実施して治療が円滑におこなわれるように支援します。初回処方時には医師の診察前に面談し、適切な薬剤について処方提案しています。
各チーム医療への参画
チーム医療の一員として様々な場面で活躍しています。
外来化学療法チーム
抗がん薬の処方チェック、レジメン管理、無菌調製、患者さんへの副作用説明等を行い、安全で安心できるがん化学療法の実施に貢献しています。また、保険薬局と連携し、外来での抗がん薬治療を安全に継続できるよう支援しています。
HIV・AIDSサポートチーム
服薬方法に注意が必要な薬や特有の副作用を持つ薬、大きくて服用しづらい薬などを生涯に渡り長期間服薬しなければならない事により生じる精神的不安、身体的障害などに対して他職種と連携をとりながら様々なサポートを行っています。
緩和ケアチーム
緩和ケア外来での診察に同席し、症状緩和に関する処方提案を医師に行い、患者さんに服薬指導を行います。また、緩和ケアチームにコンサルトされた入院患者さんにおいては、チームメンバーとともに日々の症状を把握し、さまざまな臨床上の問題に対して薬学的見地から介入し症状緩和に努めています。
栄養管理チーム(NST)
全ての患者さんの治療基盤となる栄養管理が適切に行われるように、他職種と力を結集して活動しています。コアチームカンファレンスや病棟のサテライトNSTでは静脈栄養のプランニング、電解質管理、合併症等について薬学的視点から提案し、教育活動にも参加しています。
感染管理チーム(ICT)
抗菌薬や消毒薬の適正使用への助言、使用統計、TDMなど薬剤師の専門性を活かして院内感染対策に努めています。
抗菌薬適正使用支援チーム(AST)
専任薬剤師を配置し、抗菌薬の適正使用の推進など感染症治療が最適となるようサポートしています。
リスクマネジメント
医療の質と安全性の向上をめざして、医療安全管理室の活動に参加しています。薬剤部内でのリスク事例報告のみならず、院内での薬物治療にかかるあらゆるリスク事例における検討・対策の立案にも関わっています。
糖尿病療養指導チーム
糖尿病患者さんが治療に前向きに取り組み、自己管理できるよう支援しています。カンファレンスへの参加や他職種との情報交換、回診への同行などにより、治療方針や患者さんの状態を十分把握し、治療全般にわたって支援できるように取り組んでいます。
心臓リハビリテーションチーム
循環器系薬剤の使用目的と使用上の注意点を分かりやすく説明しています。カンファレンスに参加し、他職種と連携をとりながら効果的な薬物治療を行えるように努めています。
心不全チーム
末期心不全患者さんを対象に、患者さんだけでなくご家族など周囲の環境も考慮して、多職種で退院後を見据えた治療方針を検討しています。薬剤師は患者さんに適した薬物療法を提案し、安全に治療が継続できるように努めています。
間質性肺炎サポートチーム
間質性肺炎患者さんを対象に、多職種で連携して治療を支援しています。薬剤師は外来で抗線維化薬などの薬物療法を始める患者さんやご家族に、服薬の注意点や副作用とその対策について説明し、副作用モニタリングを行いながら治療継続をサポートしています。
精神科リエゾンチーム
せん妄・認知症における周辺症状・抑うつ等の精神症状を有する入院患者さんに対し、多職種で退院までを見据えた薬物治療・心理教育などで順調に治療が継続できるようサポートしています。
認知症ケアチーム
認知症によりケアに難渋する入院患者さんに対し、多職種でサポートしています。薬剤師は多剤併用やせん妄予防のための睡眠薬の使い方、痛みのコントロールなど、ケアの手助けになるような薬物療法を提案しています。
術後疼痛管理(APS)チーム
手術後の疼痛や嘔気などの症状緩和を目的として、麻酔科医や看護師と共に多職種で活動しています。
CAR-T細胞療法チーム
白血病やリンパ腫の新たな治療法である「CAR-T細胞療法」を、医師や臨床検査技師、臨床工学士、看護師、事務職とともに行っています。薬剤師は細胞調製や細胞製品の凍結管理の他、チーム内の情報共有や調整、メーカーの製造枠確保等、コーディネーターの役割も担っています。
骨折リエゾンサービスチーム
2022年より整形外科医を中心に、大腿骨近位部骨折の患者さんを対象とした新たな多職種のチームが設立されました。薬剤師はチームの中で、二次性骨折予防のための骨粗鬆症治療薬の導入について、医師と協働しています。また、リハビリ転院される場合も多く、転院先の医療機関で治療薬を継続できるように情報共有等を行っています。
造血幹細胞移植チーム
医師、看護師、理学療法士、管理栄養士など多職種で連携し、移植患者さんの早期退院を目指して、移植カンファレンスを行っています。薬剤師は、抗がん薬や免疫抑制薬、抗菌薬、抗ウイルス薬など、すべてのお薬が安全かつ効果的に継続できるよう情報共有に取り組んでいます。
ポリファーマシー対応
一般的に使用する薬が多くなると、副作用など問題が発生しやすくなります。薬剤師は、医師や看護師と連携して入院患者さんの普段使用している薬の適正化に努めています。
IBD(炎症性腸疾患)チーム
当院では、2024年10月にIBD治療を充実させるためにIBDセンターを設置しました。薬剤師は、外来での治療を安全かつ効果的に継続できるように治療薬変更時の薬効や副作用などの説明、自己注射へ移行する際の手技説明などに関わっています。
薬剤師教育と臨床研究
薬学実務実習から新人職員の卒後教育ならびに薬剤師レジデント教育プログラム、そして専門薬剤師の育成へとシームレスな教育体制を構築しています。実習生の指導については、薬剤師レジデントならびに2年目の正規職員薬剤師がプリセプターとして学生を指導するとともに、薬剤師レジデント、職員薬剤師には経験年数の近い薬剤師がメンターとしてサポートし、それを認定実務実習指導薬剤師が統括指導する屋根瓦教育体制を実践しています。薬剤師レジデント、職員薬剤師が実習生の指導を担当することで、スタッフ全員が『ともに学び、ともに育つ』環境を大切にしています。
さらに、神戸学院大学大学院薬学研究科と教育および研究において相互に情報交換・指導を行う体制を構築しており、タスク・シフト/シェアやデジタル化の評価に関する研究、薬物動態学/薬力学(PK/PD)に基づく薬物血中濃度評価や至適投与計画の支援に関する研究に取り組み、研究プロセスを業務に反映させることで薬物治療の質と安全向上につなげていきます。
レジデント制度
概要 研修規程 研修プログラムレジデント・職員を 希望される方へ
・『自治体病院で働く病院薬剤師の紹介』
https://www.jmha.or.jp/jmha/contents/info/351
薬剤部の見学について
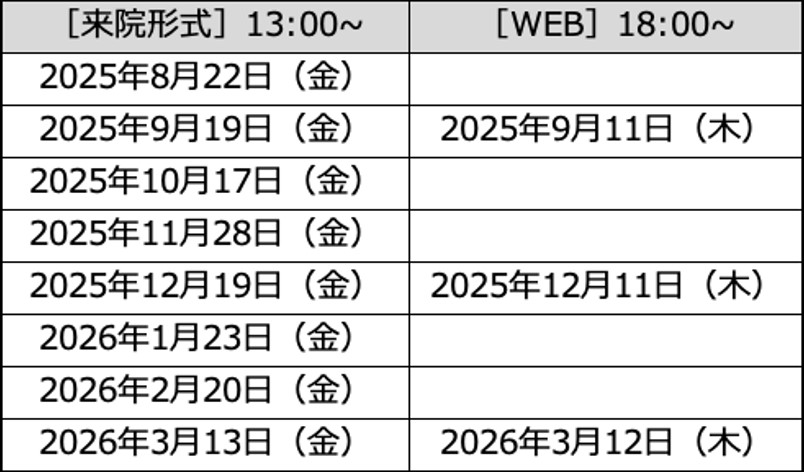
当院薬剤部では、薬剤師レジデントおよび職員を希望される方対象とした来院形式の見学会とオンライン見学を実施しております。
見学対応の年間スケジュールは以下の通りとなっております。
皆様のご参加をお待ちしております。
2025年度見学対応スケジュール
内容
来院形式:スライドを用いた説明+院内見学+質疑応答 (所要時間:2時間程度)
オンライン形式:スライドを用いた説明+質疑応答 (所要時間:1時間程度)
※見学月の希望については人数により調整させていただくことがあります。
予めご了承ください。
申込方法
下記内容を記載のうえ、見学日の14日前までにEmailにてお申し込みください。
その後、入力フォームを別途返信させていただきますのでご入力のほどよろしくお願いいたします。
- 氏名(フリガナ)
- 大学名、学年
申込先
c_pharm-publicity@kcho.jp(件名は、【見学申込】としてください。)
その他、ご不明な点などございましたら下記までお問い合わせください。
SNSについて
SNSでも当院薬剤部の情報発信を行っております。
Instagram:kcho_pharm (神戸市立医療センター中央市民病院 薬剤部)
Facebook:神戸市立医療センター中央市民病院 薬剤部
是非情報収集にご活用ください。
お問い合わせ先
担当
薬剤部 広報・見学担当 平野 達也
Tel. 078-302-4321(代表)
地域医療機関の方へ
保険薬局の方へ
院外処方せんにおける疑義照会について
院外処方せんにおける疑義照会は、
- 処方内容については薬剤部を通して医師へ
- 保険など、処方せんについては会計(医事課)へ
お問い合わせ下さい。処方医は診察中の場合がありますので、疑義内容はまとめてからお問い合わせください。
服薬情報提供書(トレーシングレポート)について
当院では、服薬情報提供書(トレーシングレポート)を用意しています。
保険薬局で「即時性は低いものの処方医師へ情報提供した方が望ましい」と判断された内容をFAX(薬剤部宛)にて集約し、医師へ情報伝達を行い情報の共有化を図っています。
下記、添付の運用についてをご確認の上、服薬情報提供書をご利用ください。
なお、外来がん化学療法に関しては、下記「外来がん化学療法に関する薬剤情報提供書」からご利用ください。
FAX送付先
(078)302-5534 薬剤部 宛て
後発医薬品へ変更後の当院への情報提供について
従来のFAXによる情報提供から、下記の方法に変更いたします。
- 後発医薬品へ変更後、患者さんに説明書をお渡しいただくか、お薬手帳に変更内容を明記していただくようお願いいたします。
- 患者さんが次回当院を受診される際に、これらを医師に見せていただけるよう、ご指導をお願いいたします。
新規採用及び削除医薬品
採用薬品一覧(2026年1月現在)令和8年1月6日 新規採用薬品及び変更・削除薬品
採用薬品
- オンジェンティス錠25㎎
- グラニセトロン内服ゼリー2㎎「ケミファ」
- ネクセトール錠180㎎
- ベピオウォッシュゲル5%(院外専用)
- ヒルドイドローション0.3% 150gボトル(ポンプ付)(院外専用)
- ロスバスタチンOD錠5㎎「DSEP」
- パキロビッドパック600
- パキロビッドパック300
- ラゲブリオカプセル200㎎
- ゾコーバ錠125㎎(院外専用)
削除薬品
- トロペロン錠1mg(院外専用)
令和7年12月2日 新規採用薬品及び変更・削除薬品
採用薬品
- クラシエ加味帰脾湯エキス細粒(院外専用)
- クラシエ加味逍遙散料エキス細粒(院外専用)
- クラシエ桂枝茯苓丸料エキス細粒(院外専用)
- クラシエ柴胡桂枝湯エキス細粒(院外専用)
- クラシエ柴苓湯エキス細粒(院外専用)
- クラシエ十全大補湯エキス細粒(院外専用)
- クラシエ人参養栄湯エキス細粒(院外専用)
- クラシエ桃核承気湯エキス細粒(院外専用)
- クラシエ当帰芍薬散料エキス細粒(院外専用)
- クラシエ半夏瀉心湯エキス細粒(院外専用)
- クラシエ補中益気湯エキス細粒(院外専用)
- クラシエ抑肝散加陳皮半夏エキス細粒(院外専用)
- マグミット細粒83%(院外専用)
- ツムラ抑肝散加陳皮半夏エキス顆粒(医療用) (院外専用)
- ツムラ清暑益気湯エキス顆粒(医療用) (院外専用)
- ツムラ黄耆建中湯エキス顆粒(医療用) (院外専用)
- ツムラ当帰建中湯エキス顆粒(医療用) (院外専用)
- ツムラ当帰芍薬散エキス顆粒(医療用) (院外専用)
- アリドネパッチ27.5mg(院外専用)
- アリドネパッチ55mg(院外専用)
- ヘパリン類似物質外用泡状スプレー0.3%「ニットー」(院外専用)
削除薬品
- ヘパリン類似物質外用泡状スプレー0.3%「日本臓器」
令和7年11月5日 新規採用薬品及び変更・削除薬品
採用薬品
- ドプテレット錠20mg
- ウゴービ皮下注0.25mgペン 1.0MD
- ウゴービ皮下注0.5mgペン 2.0MD(院外専用)
- ウゴービ皮下注1.0mgペン4.0MD(院外専用)
- ウゴービ皮下注1.7mgペン6.8MD(院外専用)
- ウゴービ皮下注2.4mgペン 9.6MD(院外専用)
- ブルキンザカプセル80mg(院外専用)
- ベルスピティ錠2mg(院外専用)
- コンドロイチン点眼液3%「日点」(院外専用、要時)
- レボカバスチン点眼液0.025%「TS」(院外専用)
- フルバスタチン錠30mg「サワイ」(院外専用)
- フルバスタチン錠20mg「サワイ」(要時) パスタロンローション10% 50g
削除薬品
- アイドロイチン3%点眼液(院外専用、要時)
- リボスチン点眼液0.025%
- ローコール錠30mg(院外専用)
- ローコール錠20mg(要時)
- ウレパールローション10% 20g
令和7年10月15日 新規採用薬品及び変更・削除薬品
採用薬品
- ウェリレグ錠40mg g(院外専用、要時)
- ジンタス錠50mg(院外専用)
はじめに
ここに公開するレジメンは、神戸市立医療センター中央市民病院 がん化学療法委員会で審査、承認されたのもです。本資料は当院でがん治療を受けられる患者の適正な投与管理を目的として提供するものであり、その他の目的での用途は想定しておりません。投与量、投与スケジュール、支持療法は患者の状態によって変更される場合があります。
がん化学療法レジメン
がん化学療法レジメン 肺癌
更新日時:2025年3月14日
| P16-101 | (放治あり)PTX weekly | |
| P16-101 | (放治なし)PTX weekly | |
| P15-204 | 3週毎 S1/CDDP | |
| A15-202 | Abraxane (d1,8,15)/CBDCA triweekly | |
| A15-101 | Abraxane weekly | |
| P15-404 | ADOC | |
| B17-202 | Afatinib daily/Bev triweekly | |
| A21-101 | AMR(35)(d1-3)triweekly(NSCLC) | |
| A21-101 | AMR(40)(d1-3)triweekly(SCLC) | |
| A19-302 | Atezo/CBDCA(5)/ETP(100)(d1-3)q3w | |
| A19-302 | Atezo/CBDCA/nabPTX(d1,8,15) q3w | |
| A19-304 | Atezo/CDDP/PEM q3w | |
| A19-202 | Atezo/PEM q3w 維持療法 | |
| A19-303 | Atezo/PEM/CBDCA q3w | |
| A18-102 | Atezolizumab triweekly(NSCLC) | |
| A18-202 | Atezolizumab/Bev q3w 維持療法 | |
| A18-402 | Atezolizumab/Bev/CBDCA/PTX q3w | |
| B15-102 | Bev triweekly | |
| A15-303 | Bev/Abraxane (d1,8,15)/CBDCA triweekly | |
| D16-202 | Bev/DTX triweekly | |
| G15-202 | Bev/GEM(d1,8) triweekly | |
| P15-203 | Bev/PEM(500) triweekly | |
| P15-304 | Bev/PEM/CBDCA triweekly | |
| P15-306 | Bev/PEM/CDDP triweekly | |
| P16-202 | Bev/PTX (d1,8,15) monthly | |
| P16-303 | Bev/PTX(d1,8,15)/CBDCA monthly | |
| P16-303 | Bev/PTX/CBDCA triweekly | |
| C19-101 | CBDCA(30) daily+RT (NSCLC) | |
| C15-203 | CBDCA(5)/ETP(80)(d1-3)triweekly | |
| P15-101 | CDDP it | |
| P15-201 | CDDP it/ADM it | |
| P15-205 | CDDP(80)/ETP(100)(d1-3)triweekly | |
| P15-201 | CDDP(d1-3)/ETP(d1-3)triweekly | |
| V15-403 | CODE 第1週目 | |
| V15-202 | CODE 第2,4,6,8週目 | |
| A15-303 | CODE 第3,5,7,9週目 | |
| CPT16-102 | CPT-11(100) weekly(肺癌) | |
| CPT15-203 | CPT-11(d1,8,15)/CBDCA monthly | |
| CPT15-205 | CPT-11(d1,8,15)/CDDP monthly(NSCLC) | |
| CPT15-204 | CPT-11(d1,8,15)/CDDP monthly(SCLC) | |
| D16-101 | DTX(60) triweekly(肺癌) | |
| D16-202 | DTX(d1,8)/CDDP(d1,8) monthly | |
| D16-202 | DTX/CBDCA triweekly(肺癌) | |
| D16-203 | DTX/CDDP triweekly | |
| D20-302 | Durva(BW≦30)/CBDCA/ETP(d1-3)q3w | |
| D20-303 | Durva(BW≦30)/CDDP/ETP(d1-3)q3w | |
| D23-101 | Durva(Treme/Durva)q4w 維持初回・3回目~ | |
| D20-302 | Durva/CBDCA/ETP(d1-3)q3w | |
| D20-303 | Durva/CDDP/ETP(d1-3)q3w | |
| D23-201 | Durva/PEM(Treme/Durva)q4w 維持初回・3回目~ | |
| D23-101 | Durvalumab after CRT q4w(BW>30kg)(NSCLC) | |
| D23-101 | Durvalumab after CRT q4w(BW≦30kg)(NSCLC) | |
| D20-101 | Durvalumab(BW≦30) 維持 q4w(ES-SCLC) | |
| D20-101 | Durvalumab 維持 q4w(ES-SCLC) | |
| B15-202 | Erlotinib daily/Bev triweekly | |
| E15-102 | ETP(d1-3) triweekly | |
| B17-202 | Gefitinib daily/Bev triweekly | |
| G15-101 | GEM(d1,8) triweekly(肺癌) | |
| G15-202 | GEM(d1,8)/CBDCA(AUC=4-6) triweekly | |
| G15-204 | GEM(d1,8)/CDDP triweekly | |
| G15-101 | GEM(d1,8,15)monthly (肺癌) | |
| G19-304 | Neci(d1,8)/GEM(d1,8)/CDDP triweekly | |
| N19-101 | Necitumumab(d1,8) triweekly 維持療法 | |
| N23-301 | Nivo/GEM(d1,8)/CDDP q3w(術前)(扁平上皮癌) | |
| N20-203 | Nivo(d1,22)/Ipi(d1) q6w | |
| N20-405 | Nivo/Ipi/PEM/CBDCA q3w(1c目) | |
| N20-405 | Nivo/Ipi/PEM/CDDP q3w(1c目) | |
| N20-404 | Nivo/Ipi/PTX/CBDCA q3w(1c目) | |
| N20-304 | Nivo/PEM/CBDCA q3w(2c目) | |
| N23-301 | Nivo/PEM/CDDP triweekly(術前)(非扁平上皮癌) | |
| N20-304 | Nivo/PEM/CDDP q3w(2c目) | |
| N20-303 | Nivo/PTX/CBDCA q3w(2c目) | |
| N23-301 | Nivo/PTX/CBDCA triweekly(術前) | |
| N18-102 | Nivolumab biweekly (NSCLC) | |
| N20-102 | Nivolumab(480mg/b) q4w (NSCLC) | |
| O24-201 | Osimertinib+PEM triweekly (維持) | |
| O24-301 | Osimertinib+PEM/CBDCA triweekly | |
| O24-301 | Osimertinib+PEM/CDDP triweekly | |
| P15-102 | PEM(500) triweekly | |
| P15-203 | PEM/CBDCA triweekly | |
| P15-206 | PEM/CDDP triweekly | |
| P17-102 | Pembrolizumab triweekly(NSCLC) | |
| P20-101 | Pembrolizumab(400mg/b) q6w(NSCLC) | |
| P18-302 | Pembrolizumab/CBDCA/nabPTX(d1,8,15)q3w | |
| P18-303 | Pembrolizumab/CBDCA/PEM q3w 1-4c | |
| P18-302 | Pembrolizumab/CBDCA/PTX triweekly | |
| P24-301 | Pembrolizumab/CDDP/PEM q3w(術前) | |
| P18-304 | Pembrolizumab/CDDP/PEM q3w 1-4c | |
| P24-301 | Pembrolizumab・GEM(d1,8)/CDDP q3w (術前) | |
| P18-202 | Pembrolizumab/PEM q3w 維持療法 | |
| P16-202 | PTX(70)(d1,8,15)/CBDCA(6) monthly | |
| P16-202 | PTX/CBDCA triweekly (肺癌) | |
| P16-201 | PTX/CBDCA weekly | |
| R18-102 | Ram triweekly | |
| D16-202 | Ram/DTX triweekly | |
| E20-201 | Ram/Erlotinib(d1-14)biweekly | |
| B17-202 | S1(d1-14)/Bev triweekly(肺癌) | |
| C15-202 | S1(d1-14)/CBDCA triweekly | |
| T23-101 | T-DXd triweekly (肺癌) | |
| T15-101 | Topotecan(d1-5) triweekly | |
| T23-401 | Treme/Durva/nabPTX(d1,8,15)・CBDCA q3w 1-4c | |
| T23-401 | Treme/Durva/PEM/CBDCA triweekly 1-4c | |
| T23-401 | Treme/Durva/PEM/CDDP triweekly 1-4c | |
| T23-201 | Treme/Durva q4w 維持2回目 | |
| T23-301 | Treme/Durva/PEM q4w 維持2回目 | |
| V15-101 | VNR weekly (肺癌) | |
| V15-202 | VNR(d1,8)/CBDCA triweekly | |
| V15-204 | VNR(d1,8)/CDDP triweekly | |
| V15-201 | VNR(d1,8)/CDDP(d1,8) triweekly | |
| P15-204 | 放射線併用 3週毎 S1/CDDP | |
| V15-202 | 放射線併用VNR(d1,8)/CDDP(d1,8)triweekly |
がん化学療法レジメン 乳癌
更新日時:2025年3月14日
| A15-101 | Abraxane triweekly | |
| A15-201 | AC(乳癌) | |
| A19-201 | Atezo(d1,15)/nabPTX(d1,8,15) monthly | |
| F15-201 | CMF(乳癌) | |
| CPT16-102 | CPT-11 weekly(乳癌) | |
| A15-202 | dose-dense AC(乳癌) | |
| E22-201 | dose-dense EC(乳癌) | |
| D16-101 | DTX triweekly(乳癌) | |
| D16-201 | DTX/CPA triweekly(乳癌) | |
| E15-202 | EC triweekly(乳癌) | |
| E15-101 | Eribulin (d1,8) triweekly | |
| F15-301 | FEC(乳癌) | |
| G15-101 | GEM (d1,8) triweekly(乳癌) | |
| F15-301 | iv CMF(乳癌) | |
| P22-301 | Pembro/ADM(60)/CPA(600) triweekly | |
| P22-301 | Pembro/EPI(90)/CPA(600) triweekly | |
| P22-301 | Pembro/wPTX(80)/CBDCA (AUC5) triweekly | |
| P21-301 | Pembro/GEM/CBDCA(AUC=2) triweekly | |
| P22-101 | Pembrolizumab (200) q3w(乳癌) | |
| P22-101 | Pembrolizumab (400) q6w(乳癌) | |
| P21-201 | Pembrolizumab/nab-PTX(d1,8) triweekly | |
| P21-201 | Pembrolizumab/PTX(d1,8) triweekly | |
| P18-201 | PER/Tmab triweekly (1コース目) | |
| P18-201 | PER/Tmab triweekly(2コース目以降) | |
| P18-301 | PER/Tmab/DTX triweekly (1コース目) | |
| P18-301 | PER/Tmab/DTX triweekly (2コース目以降) | |
| P19-401 | PER/Tmab/DTX/CBDCA triweekly(1コース目) | |
| P19-401 | PER/Tmab/DTX/CBDCA triweekly(2コース目以降) | |
| P18-301 | PER/Tmab/PTX triweekly (1コース目) | |
| P18-301 | PER/Tmab/PTX triweekly (2コース目以降) | |
| P23-201 | PHESGO triweekly (1コース目) | |
| P23-201 | PHESGO triweekly(2コース目以降) | |
| P23-301 | PHESGO/DTX triweekly(1コース目) | |
| P23-301 | PHESGO/DTX triweekly(2コース目以降) | |
| P23-401 | PHESGO/DTX/CBDCA triweekly(1コース目) | |
| P23-401 | PHESGO/DTX/CBDCA triweekly(2コース目以降) | |
| P23-301 | PHESGO/nabPTX triweekly (1コース目) | |
| P23-301 | PHESGO/nabPTX triweekly (2コース目以降) | |
| P23-301 | PHESGO/PTX triweekly (1コース目) | |
| P23-301 | PHESGO/PTX triweekly (2コース目以降) | |
| P23-302 | PHESGO/VNR(d1,8) triweekly (1コース目) | |
| P23-302 | PHESGO/VNR(d1,8) triweekly (2コース目以降) | |
| P16-101 | PTX weekly (乳癌) | |
| P16-201 | PTX(d1,8,15)/BV(d1,15) monthly | |
| T15-101 | T-DM1 triweekly | |
| T20-101 | T-DXd triweekly | |
| T20-101 | Tmab triweekly(1回目) | |
| T20-101 | Tmab triweekly(2回目以降) | |
| T20-101 | Tmab weekly(1回目) | |
| T20-101 | Tmab weekly(2回目以降) | |
| T20-201 | Tmab/Abraxane triweekly(1回目) | |
| T20-201 | Tmab/Abraxane triweekly(2回目以降) | |
| T20-203 | Tmab/CPT-11 weekly (1回目) | |
| T20-203 | Tmab/CPT-11 weekly (2回目以降) | |
| T20-201 | Tmab/DTX triweekly(1回目) | |
| T20-201 | Tmab/DTX triweekly(2回目以降) | |
| T20-301 | Tmab/DTX/CBDCA triweekly(1コース目) | |
| T20-301 | Tmab/DTX/CBDCA triweekly(2コース目以降) | |
| T20-301 | Tmab/DTX/CPA triweekly(1コース目) | |
| T20-301 | Tmab/DTX/CPA triweekly(2コース目以降) | |
| T20-201 | Tmab/Eribulin (d1,8) triweekly(1コース目) | |
| T20-201 | Tmab/Eribulin (d1,8) triweekly(2コース目以降) | |
| T20-201 | Tmab/PTX weekly(1回目) | |
| T20-201 | Tmab/PTX weekly(2回目以降) | |
| T20-301 | Tmab/PTX(d1, 8)/GEM(d1, 8) triweekly(1コース目) | |
| T20-301 | Tmab/PTX(d1, 8)/GEM(d1, 8) triweekly(2コース目以降) | |
| T20-301 | Tmab/PTX/CBDCA triweekly(1コース目) | |
| T20-301 | Tmab/PTX/CBDCA triweekly(2コース目以降) | |
| T20-201 | Tmab/VNR(d1, 8)triweekly(1コース目) | |
| T20-201 | Tmab/VNR(d1, 8)triweekly(2コース目以降) | |
| V15-101 | VNR weekly (乳癌) | |
| T20-201 | weekly Tmab/Abraxane (2回目以降) |
がん化学療法レジメン 胃癌
更新日時:2025年3月14日
| P15-203 | 5週毎 S1/CDDP | |
|---|---|---|
| A17-101 | Abraxane weekly(胃癌) | |
| CPT15-103 | CPT-11 biweekly (胃癌) | |
| CPT15-202 | CPT-11(d1, 15)/CDDP monthly(胃癌) | |
| CPT15-203 | CPT-11/CDDP biweekly | |
| D16-101 | DTX weekly (胃癌) | |
| F17-203 | FOLFOX6(胃癌) | |
| F21-201 | 5-FU/l-LV+PTX monthly | |
| O15-202 | G-SOX (100mg/m2) triweekly | |
| O15-202 | G-SOX (130mg/m2) triweekly | |
| O15-202 | G-XELOX triweekly | |
| H18-201 | HER/CAP(d1-14) triweekly(1回目) | |
| H18-201 | HER/CAP(d1-14) triweekly(2回目以降) | |
| O18-301 | HER/G-SOX triweekly (1コース目) | |
| O18-301 | HER/G-SOX triweekly (2コース目以降) | |
| O18-301 | HER/XELOX triweekly (1コース目) | |
| O18-301 | HER/XELOX triweekly (2コース目以降) | |
| H18-302 | HER/XP(1回目) | |
| H18-302 | HER/XP(2回目以降) | |
| T20-403 | mFOLFOX6+Tmab biweekly(1c目) | |
| T20-403 | mFOLFOX6+Tmab biweekly(2c目以降) | |
| P18-203 | nabPTX(d1,8,15)/Ram(d1,15) monthly | |
| N21-303 | Nivo/SOX triweekly | |
| N21-303 | Nivo/XELOX triweekly | |
| N21-303 | Nivo+mFOLFOX6 biweekly | |
| N18-102 | Nivolumab biweekly (胃癌) | |
| N20-102 | Nivolumab(480mg/b) q4w (胃癌) | |
| P16-101 | PTX weekly (胃癌) | |
| R16-202 | Ram(d1,15)/PTX(d1,8,15) monthly | |
| R21-203 | Ram/CPT-11 biweekly | |
| R15-101 | Ramucirumab biweekly | |
| D16-201 | S1(d1-14)/DTX triweekly | |
| T20-102 | T-DXd triweekly(胃癌) | |
| T20-201 | Tmab/CAP(d1-14) triweekly(1回目) | |
| T20-201 | Tmab/CAP(d1-14) triweekly(2回目以降) | |
| O20-303 | Tmab/G-SOX triweekly (1コース目) | |
| O20-303 | Tmab/G-SOX triweekly (2コース目以降) | |
| P18-305 | Tmab/SP (1コース目) | |
| P18-305 | Tmab/SP (2コース目以降) | |
| O20-303 | Tmab/XELOX triweekly (1コース目) | |
| O20-303 | Tmab/XELOX triweekly (2コース目以降) | |
| T20-304 | Tmab/XP(1回目) | |
| T20-304 | Tmab/XP(2回目以降) | |
| Z24-303 | Zolbe+CAPOX triweekly 2コース目以降 | |
| Z24-301 | Zolbe(d1)+CAPOX(d2) triweekly 2コース目以降 | |
| Z24-403 | Zolbe+mFOLFOX6 biweekly 2コース目以降 | |
| Z24-401 | Zolbe(d1)+mFOLFOX6(d2) biweekly 2コース目以降 | |
| CPT15-203 | 胃癌CPT-11(d1, 15)/CDDP monthly |
がん化学療法レジメン 食道癌
更新日時:2024年4月4日
| F16-204 | CDGP/5FU(96時間)monthly(インフューザー) | |
| F16-303 | DCF monthly(インフューザー) | |
| F21-303 | DCF monthly(インフューザー)(ラインキープ有) | |
| F22-301 | DCF(70-70-3750) triweekly(腫内) | |
| D16-101 | DTX triweekly(食道癌) | |
| P21-201 | FP(1000/75)(5-FU:96時間,インフューザー)monthly | |
| P21-201 | FP(1000/75)(5-FU:96時間,輸液ポンフ)monthly | |
| F17-202 | FP (70-700) radiation | |
| F17-202 | high dose FP | |
| F15-203 | high dose FP(5-FU:120時間, インフューザー) | |
| F15-203 | high dose FP(5-FU:96時間, インフューザー)monthly | |
| N22-201 | Nivo(d1,22)/Ipi(d1) q6w(食道癌) | |
| N22-301 | Nivo(day1,15)/FP monthly(食道癌) | |
| N20-102 | Nivolumab biweekly (食道癌) | |
| N20-102 | Nivolumab(480mg/b) q4w (食道癌) | |
| P21-101 | Pembrolizumab triweekly(食道癌) | |
| P21-101 | Pembrolizumab(400mg/b) q6w(食道癌) | |
| P21-301 | Pembrolizumab/FP triweekly(食道癌) | |
| P16-101 | PTX weekly (食道癌) |
がん化学療法レジメン 大腸癌
更新日時:2025年3月14日
| F18-303 | Aflibercept/FOLFIRI biweekly | |
| F17-305 | Bev(10mg/kg)/FOLFOX6 biweekly | |
| F17-304 | Bev(5mg/kg)/FOLFIRI biweekly | |
| F17-305 | Bev(5mg/kg)/FOLFOX6 biweekly | |
| CPT15-304 | Bev(5mg/kg)/IRIS | |
| B15-202 | Bev(d1、15) /TAS-102(d1-5、8-12) monthly | |
| C15-203 | Bev/Cape(d1-14) triweekly | |
| O17-404 | Bev/FOLFOXIRI | |
| O15-303 | Bev/SOX triweekly | |
| O15-303 | Bev/XELOX triweekly | |
| Cm15-101 | Cmab weekly( 初回) | |
| Cm15-101 | Cmab weekly(2回目以降) | |
| C15-205 | Cmab weekly/CPT-11 biweekly(1コース目) | |
| C15-205 | Cmab weekly/CPT-11 biweekly(2コース目以降) | |
| F17-304 | Cmab(d1, 8)/FOLFIRI(1コース目) | |
| F17-304 | Cmab(d1, 8)/FOLFIRI(2コース目以降) | |
| F17-303 | Cmab(d1, 8)/FOLFOX6(1コース目) | |
| F17-303 | Cmab(d1, 8)/FOLFOX6(2コース目以降) | |
| C21-301 | C-mab+encorafenib+binimetinib ( 初回) | |
| C21-301 | C-mab+encorafenib+binimetinib (2回目以降) | |
| CPT15-103 | CPT-11 biweekly (大腸癌) | |
| F17-203 | FOLFIRI | |
| F17-202 | FOLFOX6 | |
| O17-303 | FOLFOXIRI | |
| CPT15-203 | IRIS | |
| N20-102 | Nivo(480mg,Nivo/Ipi維持q4w,(MSI-H結腸・直腸) | |
| N20-102 | Nivo(Nivo/Ipi維持 q2w(MSI-High結腸・直腸) | |
| N20-203 | Nivo/Ipi triweekly(MSI-High結腸・直腸癌) | |
| N20-102 | Nivolumab q2w (MSI-High/結腸・直腸癌) | |
| Pm16-101 | Pmab biweekly | |
| Pm15-203 | Pmab/CPT-11 biweekly | |
| F17-303 | Pmab/FOLFIRI biweekly | |
| F17-302 | Pmab/FOLFOX6 biweekly | |
| F17-304 | Ram/FOLFIRI biweekly | |
| B15-202 | S1(d1-28) / Bev(d1,15,29) 6週毎 | |
| B17-304 | SIRB triweekly | |
| O15-202 | SOX | |
| F19-101 | WHF療法 ia(インフューザーポンプ) weekly | |
| CPT18-304 | XELIRI + Bev triweekly | |
| O15-202 | XELOX |
がん化学療法レジメン 胆・肝・膵癌
更新日時:2025年3月14日
| A20-201 | Atezolizumab/Bev q3w(肝細胞癌) | |
| D23-301 | Durva(d1)/GC(d1,8) triweekly (BW>30kg) | |
| D23-301 | Durva(d1)/GC(d1,8) triweekly (BW≦30kg) | |
| D23-101 | Durva(Treme/Durva)2コース目以降(BW>30kg)q4w | |
| D23-101 | Durva(Treme/Durva)2コース目以降(BW≦30kg)q4w | |
| D23-101 | Durvalumab維持 monthly(BW>30kg)(胆道癌) | |
| D23-101 | Durvalumab維持 monthly(BW≦30kg)(胆道癌) | |
| F17-303 | FOLFIRINOX | |
| G15-101 | GEM biweekly(膵癌) | |
| G15-101 | GEM(d1,8) triweekly(胆道癌) | |
| G15-101 | GEM(d1,8) triweekly(膵癌) | |
| G15-201 | GEM(d1,8)/CDDP(d1,8)triweekly | |
| G20-201 | GEM(d1,8)+S-1(d1-14) triweekly(膵癌) | |
| G15-101 | GEM(d1,8,15)monthly (胆道癌) | |
| G15-101 | GEM(d1,8,15)monthly (膵癌) | |
| F17-304 | mFOLFIRINOX | |
| G15-204 | nabPTX(d1,8,15)/GEM(d1,8,15) | |
| I20-202 | nal-IRI/5-FU/l-LV biweekly | |
| P24-301 | Pembro(d1)/GC(d1,8) triweekly | |
| P24-301 | Pembro(d1)/GC(d1,8,22,29) q6w | |
| P24-201 | Pembro(d1)/GEM(d1,8) q3w 維持 (胆道癌) | |
| P24-201 | Pembro(d1)/GEM(d1,8,22,29)q6w 維持(胆道癌) | |
| T23-201 | Tremelimumab/Durva 1コース目(BW>30kg)monthly | |
| T23-201 | Tremelimumab/Durva 1コース目(BW≦30kg)monthly | |
| R19-102 | Ram biweekly(肝細胞癌) |
がん化学療法レジメン 婦人科癌
更新日時:2025年3月14日
| A15-101 | ACTD biweekly | |
| A15-203 | ADM/CDDP triweekly | |
| B15-305 | BEP療法(d1-5,9,16)(卵巣胚細胞腫瘍) | |
| B20-302 | Bev biweekly/CBDCA/PLD monthly | |
| B21-202 | Bev biweekly/PLD monthly | |
| B15-102 | Bev triweekly(婦人科癌) | |
| B15-202 | Bev triweekly/PLD monthly 1コース目 | |
| B15-202 | Bev triweekly/PLD monthly 2コース目 | |
| B15-202 | Bev triweekly/PLD monthly 3コース目 | |
| B16-203 | Bev triweekly/PTX weekly 1コース目 | |
| B16-203 | Bev triweekly/PTX weekly 2コース目 | |
| B16-203 | Bev triweekly/PTX weekly 3コース目 | |
| D19-302 | Bev/DTX/CBDCA triweekly(卵巣癌) | |
| B17-202 | Bev/GEM (d1,8) triweekly | |
| G15-302 | Bev/GEM(d1,8)/CBDCA tri (婦人科癌) | |
| B22-202 | Bev/Olaparib triweekly(卵巣癌) | |
| P16-303 | Bev/PTX/CBDCA triweekly(子宮頚癌) | |
| P16-303 | Bev/PTX/CBDCA triweekly(卵巣癌) | |
| B18-302 | Bev/PTX/topotecan(d1-3) triweekly | |
| B15-202 | Bev/Topotecan(d1-5) triweekly | |
| C15-101 | CBDCA weekly(婦人科癌) | |
| P15-102 | CDDP weekly(CCRT用) | |
| C15-101 | CDGP monthly | |
| C23-101 | Cemiplimab triweekly | |
| CPT15-102 | CPT-11 weekly(婦人科癌) | |
| C15-204 | CPT-11(d1,8,15)/CDDPmonthly(婦人科癌) | |
| CPT15-202 | CPT-11(d1,8)/CDGP monthly | |
| CPT15-203 | CPT-11(d1,8,15)/CDGP monthly | |
| P16-202 | divided weekly TC (AUC=2) | |
| P16-203 | dose-dense TC | |
| D16-101 | DTX triweekly(婦人科癌) | |
| D16-201 | DTX(d8)/GEM(d1,8)triweekly(婦人科癌) | |
| D16-201 | DTX/CBDCA triweekly(婦人科癌) | |
| D24-301 | Durva/PTX/CBDCA triweekly(子宮体癌) | |
| M15-501 | EMA/CO biweekly | |
| F17-201 | FA biweekly | |
| G15-201 | GEM(d1,8)/CBDCA(AUC=4) triweekly | |
| G15-101 | GEM(d1,8,15)monthly (卵巣癌) | |
| I18-101 | IFM triweekly | |
| I18-201 | IFM/PTX triweekly | |
| M14-101 | MTX (d1-5) im biweekly(絨毛性疾患) | |
| M14-101 | MTX im weekly (異所性妊娠) | |
| N15-101 | Nogitecan(d1-5) triweekly | |
| D24-201 | Olaparib/Durvalumab維持monthly(子宮体癌) | |
| A15-101 | PLD monthly(90mg/body以下) | |
| A15-101 | PLD monthly(90mg/body以上) | |
| P22-201 | Pembro/Bev triweekly 維持療法 | |
| P22-402 | Pembro/Bev/PTX/CBDCA triweekly | |
| P22-401 | Pembro/Bev/PTX/CDDP triweekly | |
| P21-201 | Pembro/Lenvatinib q3week(子宮体癌) | |
| P21-201 | Pembro/Lenvatinib q6week(子宮体癌) | |
| P22-301 | Pembro/PTX/CBDCA triweekly | |
| P22-302 | Pembro/PTX/CBDCA triweekly(子宮頚癌) | |
| P25-301 | Pembro/PTX/CBDCA triweekly(子宮体癌) | |
| P22-301 | Pembro/PTX/CDDP triweekly | |
| P22-101 | Pembrolizumab triweekly 維持療法(子宮頚癌) | |
| P25-101 | Pembrolizumab(400mg/b) q6w(子宮体癌) | |
| A15-201 | PLD/CBDCA monthly | |
| P16-102 | PTX weekly (婦人科癌) | |
| P16-203 | PTX/CBDCA triweekly(婦人科癌) | |
| P16-203 | PTX/CDDP triweekly(婦人科癌) | |
| P16-303 | TP/TE | |
| P25-201 | weekly TC(AUC=2) (CCRT前)(子宮頚癌) | |
| P16-203 | weekly TC (AUC=6) |
がん化学療法レジメン 泌尿器・胚細胞腫
更新日時:2024年4月4日
| A20-201 | Axitinib daily/Avelumab biweekly | |
| P19-201 | Axitinib daily/Pembrolizumab triweekly | |
| P20-201 | Axitinib daily/Pembrolizumab(400mg/b) q6w | |
| N24-201 | Cabozantinib daily/Nivolumab biweekly | |
| N24-201 | Cabozantinib daily/Nivolumab(480mg)monthly | |
| D16-201 | DTX(d8)/GEM(d1,8) tri (尿路上皮癌) | |
| E24-201 | Enfortumab vedotin(d1,8)+Pembrolizumab q3w | |
| E21-101 | Enfortumab vedotin q4week(尿路上皮癌) | |
| G15-202 | GC療法(d1,8,15) | |
| P15-203 | GC療法(d2) | |
| G15-202 | GEM(d1,8,15)/CBDCA(d1) monthly | |
| C14-202 | GEM(d1,8,15)/CBDCA(d2)monthly | |
| P24-201 | Lenvatinib daily/Pembrolizumab triweekly | |
| P24-201 | Lenvatinib daily/Pembrolizumab(400mg) q6w | |
| E15-402 | mitotane daily / EDP monthly | |
| M15-403 | M-VAC療法(午後開始) | |
| N18-202 | Nivo (Nivo/Ipi維持療 biweekly(腎細胞癌) | |
| N20-102 | Nivo(Nivo/Ipi維持療法480mg/b)q4w(腎細胞癌) | |
| N18-203 | Nivo/Ipi triweekly(腎細胞癌) | |
| N18-102 | Nivolumab biweekly(腎細胞癌) | |
| N20-102 | Nivolumab(480mg/b) q4w (腎細胞癌) | |
| P17-101 | Pembrolizumab tri(尿路上皮癌) | |
| P20-101 | Pembrolizumab(400mg/b) q6w(尿路上皮癌) | |
| C15-201 | PSL daily/Cabazitaxel triweekly | |
| D18-201 | PSL(d1-7)/DTX triweekly_(午前開始) | |
| P16-202 | PTX/CBDCA triweekly(尿路上皮癌) | |
| N24-101 | 術後adj Nivolumab biweekly(尿路上皮癌) | |
| N24-101 | 術後adj Nivolumab monthly(尿路上皮癌) | |
| P24-101 | 術後adj Pembrolizumab triweekly(腎癌) | |
| P24-101 | 術後adj Pembrolizumab(400mg) q6w(腎癌) |
がん化学療法レジメン 造血器腫瘍
更新日時:2025年3月14日
| B18-401 | ABVD(外来) | |
| A20-301 | A-CHP | |
| A15-101 | AzaC(d1-7)_monthly(外来) | |
| A19-101 | AzaC皮下注(d1-7)_monthly(外来) | |
| B22-101 | Blinatumomab在宅ポンプ用 28μg/d 72hr Dex有 | |
| B22-101 | Blinatumomab在宅ポンプ用 28μg/d 96hr Dex有 | |
| B22-101 | Blinatumomab在宅ポンプ用 28μg/d 72hr Dex無 | |
| B22-101 | Blinatumomab在宅ポンプ用 28μg/d 96hr Dex無 | |
| B22-101 | Blinatumomab在宅ポンプ用 15μg/㎡ 72h Dex有 | |
| B22-101 | Blinatumomab在宅ポンプ用 15μg/㎡ 96h Dex有 | |
| B22-101 | Blinatumomab在宅ポンプ用 15μg/㎡ 72h Dex無 | |
| B22-101 | Blinatumomab在宅ポンプ用 15μg/㎡ 96h Dex無 | |
| B22-101 | Blinatumomab在宅ポンプ用 9μg/d 72hr Dex有 | |
| B22-101 | Blinatumomab在宅ポンプ用 9μg/d 96hr Dex有 | |
| B22-101 | Blinatumomab在宅ポンプ用 9μg/d 72hr Dex無 | |
| B22-101 | Blinatumomab在宅ポンプ用 9μg/d 96hr Dex無 | |
| B22-101 | Blinatumomab在宅ポンプ用 5μg/㎡ 72hr Dex有 | |
| B22-101 | Blinatumomab在宅ポンプ用 5μg/㎡ 96hr Dex有 | |
| B22-101 | Blinatumomab在宅ポンプ用 5μg/㎡ 72hr Dex無 | |
| B22-101 | Blinatumomab在宅ポンプ用 5μg/㎡ 96hr Dex無 | |
| B17-101 | Brentuximab triweekly (BW>87) | |
| B17-101 | Brentuximab triweekly (BW≦87) | |
| A15-301 | CHOP(外来) | |
| CPT15-102 | CPT-11(d1-3) weekly | |
| D23-101 | Darinaparsin triweekly | |
| D21-203 | DBdsc療法 サイクル1-3 | |
| D21-203 | DBdsc療法 サイクル4-8 | |
| D21-203 | DBdsc療法 サイクル9以降 | |
| D18-202 | DBd療法 triweekly サイクル1 | |
| D18-202 | DBd療法 triweekly サイクル2~3 | |
| D18-202 | DBd療法 triweekly サイクル4~8 | |
| D17-201 | DBd療法 monthly サイクル9以降 | |
| D22-301 | DCyBorD療法(iv) サイクル1-2 | |
| D22-301 | DCyBorD療法(iv) サイクル3-6 | |
| D22-301 | DCyBorD療法(po) サイクル1-2 | |
| D22-301 | DCyBorD療法(po) サイクル3-6 | |
| D22-101 | DCyBorD療法 サイクル7以降 | |
| D21-203 | DKdsc療法 サイクル1 | |
| D21-203 | DKdsc療法 サイクル2 | |
| D21-203 | DKdsc療法 サイクル3-6 | |
| D21-203 | DKdsc療法 サイクル7以降 | |
| D20-201 | DKd療法 monthly サイクル1 | |
| D20-201 | DKd療法 monthly サイクル2 | |
| D20-201 | DKd療法 monthly サイクル3-6 | |
| D20-201 | DKd療法 monthly サイクル7- | |
| D17-201 | DLd療法 monthly サイクル1 | |
| D17-201 | DLd療法 monthly サイクル2 | |
| D17-201 | DLd療法 monthly サイクル3~6 | |
| D17-201 | DLd療法 monthly サイクル7以降 | |
| D21-203 | DLdsc療法 サイクル1-2 | |
| D21-203 | DLdsc療法 サイクル3-6 | |
| D21-203 | DLdsc療法 サイクル7以降 | |
| D23-201 | DPdsc療法 サイクル1-2 | |
| D23-201 | DPdsc療法 サイクル3-6 | |
| D23-201 | DPdsc療法 サイクル7以降 | |
| D20-401 | D-VMP療法 サイクル 1 | |
| D20-401 | D-VMP療法 サイクル 2~9 | |
| D20-401 | D-VMP療法 サイクル10以降 | |
| D21-403 | D-VMPsc療法 サイクル1 | |
| D21-403 | D-VMPsc療法 サイクル2-9 | |
| D21-403 | D-VMPsc療法 サイクル10以降 | |
| E19-301 | EPd 療法(1-2コース目) | |
| E19-301 | EPd 療法(3コース目以降) | |
| E16-301 | ERd 療法(1コース目) | |
| E16-301 | ERd 療法(2コース目) | |
| E16-301 | ERd 療法(3コース目以降) | |
| G15-301 | GCOP(1000)(外来) | |
| G15-301 | GCOP(750)(外来) | |
| G15-301 | GCOP(875)(外来) | |
| G15-101 | GDP_70歳以上(外来) | |
| I18-101 | Inotuzumab ozogamicin(寛解まで) | |
| I18-101 | Inotuzumab ozogamicin(寛解後) | |
| I20-302 | IPd 療法(BW≦85)(1コース目) | |
| I20-302 | IPd 療法(BW≦85)(2コース目以降) | |
| I20-302 | IPd 療法(BW86~100)(1コース目) | |
| I20-302 | IPd 療法(BW86~100)(2コース目以降) | |
| I22-201 | Isad療法 monthly サイクル1(BW≦85) | |
| I22-201 | Isad療法 monthly サイクル1(BW86-100) | |
| I22-201 | Isad療法 monthly サイクル2以降(BW≦85) | |
| I22-201 | Isad療法 monthly サイクル2以降(BW86-100) | |
| I22-301 | IsaKd療法 monthly サイクル1(BW≦85) | |
| I22-301 | IsaKd療法 monthly サイクル1(BW86-100) | |
| I22-301 | IsaKd療法 monthly サイクル2以降(BW≦85) | |
| I22-301 | IsaKd療法 monthly サイクル2以降(BW86-100) | |
| C19-201 | Kd weekly(1コース目) | |
| C19-201 | Kd weekly(2コース目以降) | |
| C18-201 | Kd療法(1コース目) | |
| C18-201 | Kd療法(2コース目以降) | |
| C18-301 | KRd療法( 1コース目) | |
| C18-301 | KRd療法( 2~12コース目) | |
| C18-301 | KRd療法(13~18コース目) | |
| M15-101 | Mogamulizumab weekly | |
| N18-102 | Nivolumab biweekly(ホジキンリンパ腫) | |
| O18-204 | Obi-Bendamustine C2-6 | |
| O18-402 | Obi-CHOP C2-6 | |
| O18-102 | Obi-CHOP C7-8 | |
| O18-302 | Obi-CVP C2-8 | |
| O18-102 | Obinutuzumab (維持・単独療法) | |
| O24-201 | Obi(d1,2,8,15)-Acalabrutinib q4w C2 | |
| O24-201 | Obi-Acalabrutinib q4w C3-7 | |
| A17-101 | PLD biweekly | |
| P24-301 | Pola-BR (BW<42kg) q3w C2-6 (外来) | |
| P24-301 | Pola-BR (BW≧42kg) q3w C2-6 (外来) | |
| P24-401 | Pola-R-CHP (BW<42kg) triweekly C2-6(外来) | |
| P24-401 | Pola-R-CHP (BW≧42kg) triweekly C2-6(外来) | |
| P22-101 | Pola-R-CHP triweekly C7-8(外来) | |
| M15-401 | POMP療法(最初の1年) | |
| P18-103 | Pralatrexate weekly | |
| R20-201 | R2(サイクル1) | |
| R20-201 | R2(サイクル2-5) | |
| R19-203 | R-Bendamustine(外来) | |
| R14-402 | R-THPCOP(外来) | |
| C19-301 | RCD (CPA div) | |
| R19-301 | RCD(CPA po) | |
| R19-401 | R-CHOP(外来) | |
| R19-301 | R-GCD(外来)triweekly | |
| R20-401 | R-GCOP(1000)(外来) | |
| R20-401 | R-GCOP(750)(外来) | |
| R19-201 | R-GDP 70歳以上(外来)triweekly | |
| R19-302 | R-GDP(外来)triweekly | |
| R19-101 | Rituximab(外来) | |
| R19-401 | R-mini CHOP(外来) | |
| C23-201 | VEN+low dose Ara-C皮下注(d1-10) monthly | |
| A15-102 | 亜ヒ酸 寛解後 | |
| A15-102 | 亜ヒ酸 寛解導入 |
がん化学療法レジメン 頭頸部癌
更新日時:2024年4月4日
| C15-203 | CBDCA/5-FU triweekly | |
| P18-102 | CDDP(40)+RT weekly | |
| Cm15-101 | Cmab weekly 放射線併用( 初回) | |
| Cm15-101 | Cmab weekly 放射線併用(2回目以降) | |
| C15-304 | Cmab(d1,8,15)/CBDCA・5-FU triweekly(初回コース) | |
| C15-304 | Cmab(d1,8,15)/CBDCA/5-FUtriweekly(2回目以降) | |
| C15-305 | Cmab/FP triweekly(初回コース) | |
| C15-305 | Cmab/FP triweekly(2コース目以降) | |
| C16-201 | Cmab/PTX weekly (1コース目) | |
| C16-201 | Cmab/PTX weekly (2コース目以降) | |
| F15-205 | FP(70-700)(5-FU・96時間,インフューザー)(頭頸部)) | |
| N18-102 | Nivolumab biweekly (頭頸部癌) | |
| N20-102 | Nivolumab(480mg/b) q4w (頭頸部癌) | |
| P19-101 | Pembrolizumab triweekly(頭頸部癌) | |
| P20-101 | Pembrolizumab(400mg/b) q6w(頭頸部癌) | |
| P19-304 | Pembrolizumab/CBDCA/5-FU triweekly | |
| P19-304 | Pembrolizumab/FP triweekly | |
| P16-101 | PTX weekly (頭頸部癌) |
がん化学療法レジメン 皮膚癌
更新日時:2023年11月29日
| D15-101 | DTIC(250mg/m2)(d1-5) monthly | |
| D16-101 | DTX triweekly (Paget病) | |
| D16-101 | DTX weekly (Paget病) | |
| I18-101 | Ipilimumab triweekly | |
| N18-203 | Nivo/Ipi triweekly(悪性黒色腫) | |
| N23-101 | Nivolumab(480mg/b) q4w (悪性黒色腫) | |
| N18-102 | Nivolumab biweekly(悪性黒色腫) | |
| P23-101 | Pembrolizumab(400mg/b) q6w(悪性黒色腫) | |
| P19-101 | Pembrolizumab triweekly(悪性黒色腫) | |
| P16-101 | PTX weekly (Paget病) |
地域医療機関の先生方へ
院外薬局と病院との連携を深め、薬物療法の安全性を確保するための情報共有を目的として、様々な研修会を開催していますので、奮ってご参加ください。また、服薬情報提供書(トレーシングレポート)を用いた情報共有により、薬薬連携を強化していきます。患者さんをご紹介いただく際は、お薬手帳の携帯や電子お薬手帳の活用をご指導いただきますようお願いいたします。
診療記録などの利用について
診療記録などの利用について
当院へ受診中の患者さんへ臨床研究の実施に関するお知らせ
現在薬剤部では、下記の臨床研究を実施しております。
この研究では、患者さんの日常診療で得られたデータ(情報)を利用させていただきます。
ご自身のデータがこの研究に利用されることについて、異議がある場合は、情報の利用をいつでも停止することができます。研究の計画や内容などについて詳しくお知りになりたい方、ご自身のデータがこの研究で利用されることについて異議のある方、その他ご質問がある方は、以下の「問い合わせ先」へご連絡ください。
| 研究題目 | 説明文(PDF) |
| フェンタニル注出荷制限に伴う代替薬としてのブプレノルフィン注の使用状況とその有用性 | |
|---|---|
| 二次骨折予防に対する骨折リエゾンサービスチームの薬剤師の介入効果 | |
| ニューモシスチス肺炎予防に対するアトバコンの効果とその関連因子の評価 | |
| ランバート・イートン重症筋無力症の治療薬を院内製剤から市販製剤に切り替えた際の治療への影響 | |
| ダリドレキサントの使用実態調査・副作用データベースを用いた副作用プロファイル解析 | |
| せん妄発症レジストリデータの構築による適正薬物療法管理の同定:多施設共同コホート研究 | |
| 肺がん治療における新規NK1受容体拮抗薬であるホスネツピタントの薬物間相互作用に関する検討 | |
| ホスレボドパ・ホスカルビドパ水和物持続皮下注射療法に対する薬剤師の関わり | |
| バンコマイシンの時間濃度曲線下面積をガイドとしたTDM(AUC-guided TDM)における薬物動態解析を利用した代替AUC推定方法の検討 | |
| 頭頸部がん患者における同時化学放射線療法による口腔咽頭粘膜炎に対するオピオイド鎮痛薬の適正使用を目指した使用状況調査と疼痛マネジメントの検討 | |
| アナフィラキシー患者におけるエピペン®使用と治療上の問題点の評価 | |
| 抗がん薬ロボット調製を考慮した単回バイアル複数回使用のための multidose vial の検討 | |
| ASTカンファレンスの開催頻度の増加が抗菌薬適正使用に及ぼす影響 | |
| 鼓室形成術における感染予防抗菌薬の適正化に対する取り組みと効果 | |
| 免疫チェックポイント阻害薬による有害事象早期発見のための薬剤師による検査入力支援の有用性に関する研究 | |
| 精神科リエゾンチームにおける薬剤師介入事例からの適正使用についての取り組み | |
| 腎機能障害患者におけるボリコナゾール注長期投与の腎機能に対する影響について | |
| 卵巣がん患者におけるPARP阻害薬の有害事象のリスク因子探索と血中濃度解析 | |
| 有機リン中毒におけるプラリドキシム投与開始までの時間とコリンエステラーゼ値回復への影響に関する調査 | |
| 糖尿病患者におけるトルソー症候群に対するリバーロキサバンの投与実態の調査 | |
| 急性期病院における冠動脈疾患に対する治療薬の実態調査 | |
| 急性期病院における中心静脈栄養施行時の脂肪乳剤併用方法についての実態調査 | |
| ICUにおけるプロポフォール投与による高トリグリセリド血症の実態調査 | |
| 肝細胞がんに対するレンバチニブの副作用による治療中止を回避するための減量休薬の意義に関する研究 | |
| ICU退室後患者の薬学的問題点の検討 | |
| まむし抗毒素によるアナフィラキシー反応予防を目的としたアドレナリン投与の有用性および安全性に関する調査 | |
| 周術期支援センターにおけるSGLT2阻害薬の薬学的管理 | |
| 実臨床における前立腺癌治療薬アパルタミドの皮膚障害の発現状況とリスク因子の探索 | |
| 入院前準備センターにおける薬剤師業務の分析とその有用性の検証 | |
| 2型糖尿病性ケトアシドーシスで救急搬送された患者の実態調査 | |
| 経口糖尿病薬 SGLT2 阻害薬が術後アシドーシスに与える影響 | |
| 造血幹細胞移植における赤血球数変動を考慮した免疫抑制剤タクロリムスの全血中濃度予測 | |
| 同種造血幹細胞移植患者におけるヘモグロビン値の変動に影響を及ぼす因子の探索 | |
| ベンダムスチン投与患者におけるリンパ球減少症のリスク因子の検討 | |
| 重症のCOVID-19患者におけるレムデシビルの薬物動態の検討 | |
| 重症のCOVID-19患者におけるファビピラビルの薬物動態の検討 | |
| バンコマイシン投与患者の治療関連データベースの構築とそれを利用した体内動態に対する影響因子の抽出および母集団パラメータの最適化の拡大調査 | |
| Hyper-CVAD/MA 療法におけるメトトレキサートの母集団薬物動態モデル解析 | |
| 胃切除後の栄養障害における六君子湯の改善効果についての後方視的調査 | |
| 日本人固形癌患者におけるMSI-High発現状況とペムブロリズマブ投与時の有効性および安全性の検討 | |
| 去勢抵抗性前立腺癌におけるエンザルタミド用量漸増法の有害事象評価 | |
| 非小細胞肺がん患者におけるドセタキセル+ラムシルマブ併用療法の好中球減少の PK/PD モデル解析 | |
| 薬毒物中毒疑い症例に対する血中濃度測定および動態解析とその有用性の評価 | |
| 急性期病院における中心静脈栄養施行時の脂肪乳剤併用が肝機能に及ぼす影響の評価 | |
| 糖尿病合併卵巣がん患者におけるdose-dense TC 療法の有効性および安全性の評価 | |
| 緩和ケアにおける統合医療としての補完代替医療(CAM)のアロマトリートメント効果の検証 | |
| スボレキサント及び他の催眠鎮静剤の転倒・転落リスクについての評価 | |
| 神戸市立医療センター中央市民病院におけるトレーシングレポート運用の課題とその対策の検討 | |
| 非小細胞肺がん患者における免疫チェックポイント阻害薬の薬物動態モデルの構築 | |
| ヒドロモルフォン注射剤から経口剤への換算についての検討 | |
| フェンタニルの併用によるワルファリンの作用増強についての検討 | |
| 肝疾患専門医療機関における肝炎医療コーディネーターとしての薬剤師の取り組み | |
| プロセス指標とアウトカム指標に基づいたAntimicrobial Stewardship Teamの介入効果 | |
| 糖尿病患者におけるがん化学療法の有効性および安全性の評価 | |
| バンコマイシン投与患者の治療関連データベースの構築とそれを利用した体内動態に対する影響因子の抽出および母集団パラメータの最適化 | |
| ダプトマイシン投与における副作用調査と副作用発現に影響を与える因子の解明 |
お知らせ
2023年度 誌上発表
著書
- 室井延之: 投与エネルギー・栄養素投与量の決定法. JSPEN栄養療法ポケットブック. 日本臨床栄養代謝学会(編集). 南江堂, 東京, 21-24, 2023
- 田村亮: エタノール. 新版 急性中毒標準診療ガイド. 日本中毒学会(監修), 日本中毒学会学術委員会/急性中毒標準診療ガイド改訂委員会(編集). へるす出版, 東京, 132-133, 2023
論文
- Tamura R, Irie K, Nakagawa A, Muroi H, Eto M, Ikesue H, Muroi N, Fukushima S, Tomii K, Hashida T: Population pharmacokinetics and exposure-clinical outcome relationship of remdesivir major metabolite GS-441524 in patients with moderate and severe COVID-19. CPT Pharmacometrics Syst Pharmacol. 12(4): 513-521, 2023
- Fujiwara M, Uchida M, Endo M, Goto M, Shimizu T: Cardiac adverse events associated with multiple myeloma patients treated with proteasome inhibitors. Oncology. 101(5): 343-348, 2023
- Irie K, Hiramoto N, Yamaoka K, Fukushima S. Cerebrospinal fluid concentration of foscarnet in patients with HHV-6 encephalitis after hematopoietic stem cell transplantation. J Antimicrob Chemother. 78(6): 1549-1551, 2023
- Yamaoka K, Fujiwara M, Uchida M, Uesawa Y, Muroi N, Shimizu T. Adverse event profile of azacitidine: analysis by route of administration using Japanese pharmacovigilance database. Oncology. 101(10): 664-674, 2023
- Takayama Y, Kusamori K, Katsurada Y, Obana S, Itakura S, Nishikawa M. Efficient delivery of mesenchymal stem/stromal cells to injured liver by surface PEGylation. Stem Cell Res Ther. 14(1): 216, 2023
- Yamaoka K, Irie K, Hiramoto N, Hirabatake M, Ikesue H, Hashida T, Shimizu T, Ishikawa T, Muroi N. Safety and blood levels of daratumumab after switching from intravenous to subcutaneous administration in patients with multiple myeloma. Invest New Drugs. 41(5): 761-767, 2023
- Tanaka F, Irie K, Fukui N, Horii R, Imamura H, Hirabatake M, Ikesue H, Muroi N, Fukushima S, Sakai N, Hashida T. Pharmacokinetics of temozolomide in a patient with glioblastoma undergoing hemodialysis: a short communication. Ther Drug Monit. 45(6): 823-826, 2023
- Dote S, Shiwaku E, Kohno E, Fujii R, Mashimo K, Morimoto N, Yoshino M, Odaira N, Ikesue H, Hirabatake M, Takahashi K, Takahashi M, Takagi M, Nishiuma S, Ito K, Shimato A, Itakura S, Takahashi Y, Negoro Y, Shigemori M, Watanabe H, Hayasaka D, Nakao M, Tasaka M, Goto E, Kataoka N, Yokomizo A, Kobayashi A, Nakata Y, Miyake M, Hayashi Y, Yamamoto Y, Hirata T, Azuma K, Makihara K, Fukui R, Tokutome A, Yagisawa K, Honda S, Meguro Y, Suzuki S, Yamaguchi D, Miyata H, Kobayashi Y. Impact of prior bevacizumab therapy on the incidence of ramucirumab-induced proteinuria in colorectal cancer: a multi-institutional cohort study. Int J Clin Oncol. 28(8): 1054-1062, 2023
- Katsura H, Suga Y, Kubo A, Sugimura H, Kumatani K, Haruki K, Yonezawa M, Narita A, Ishijima R, Ikesue H, Toi H, Takata N. Risk evaluation of proton pump inhibitors for panitumumab-related hypomagnesemia in patients with metastatic colorectal cancer. Biol Pharm Bull. 47(1): 98-103, 2024
- 高瀬友貴, 山田圭位子, 栗原広大, 田渕宏典, 尾山将樹, 橋田亨, 室井延之: 医薬品の供給不足による院外処方の問合せに対する院内対応型の簡素化プロトコールの有用性. 医療薬学 49(7): 247-253, 2023
- 佐久間美佐緒, 増本憲生, 吉岡千尋, 有吉紗絵, 吉野有香子, 奥貞智, 橋田亨, 平畠正樹, 池末裕明, 室井延之. 入院前準備センターにおける薬剤師業務時間の分析とその有用性の検証. 日本病院薬剤師会雑誌 59(6): 623-629, 2023
- 義平祥菜, 玉木理衣, 山田留美, 上武千晶, 高原美会子, 表日登美, 熊谷佐代子, 興津美由紀, 室井延之, 橋田亨: OJTプログラムに基づく評価シートを使用した教育プログラムの導入の評価. Jpn Pharmacol Ther. 51(5): 628-631, 2023
総説
- 薩摩由香里: 振り返れば国試. 第4回 在宅における医療用麻薬の服薬指導のポイントは? 月刊薬事 65(13): 2701-2706, 2023
- 池末裕明: ホルモン療法薬の副作用と支持療法. 前立腺がん治療薬による副作用 5. ホットフラシュ. 月刊薬事 2023年7月増刊号 65(10): 2057-2062, 2023
- 池末裕明: ホルモン療法薬の副作用と支持療法. 前立腺がん治療薬による副作用 6. 性欲減退・勃起障害. 月刊薬事 2023年7月増刊号 65(10): 2063-2066, 2023
- 池末裕明: ホルモン療法薬の副作用と支持療法. 前立腺がん治療薬による副作用 7. 女性化乳房. 月刊薬事 2023年7月増刊号 65(10): 2067-2070, 2023
- 池末裕明: ホルモン療法薬の副作用と支持療法. 前立腺がん治療薬による副作用 8. 肝機能障害. 月刊薬事 2023年7月増刊号 65(10): 2071-2074, 2023
- 田中裕人: 入退院支援を再点検! 退院後を見据えた薬学管理. 入院支援センターにおける薬剤師の活動. 月刊薬事 2023年10月増刊号 65(13): 22-25, 2023
- 橋田亨: 医薬品が手に入らない事態が頻発、なぜ?薬剤師はどうすべき, Be Ambitious ! 連載第12回, TURNUP 62,15, Apr.2023
- 橋田亨: 人前で話すのが苦手。どうしたら上手なプレゼンができるのですか?,Be Ambitious ! 連載第13回, TURNUP 63,15, June.2023
- 橋田亨: 生成AIの話題沸騰!薬学教育は、薬剤師の仕事はどう変わるのか?,Be Ambitious ! 連載第14回, TURNUP 64,15, Aug.2023
- 橋田亨: 日本の薬剤師の学位は「B.S.」か? 「Pharm.D. 」か?, Be Ambitious ! 連載第15回, TURNUP 65,15, Oct.2023
- 橋田亨: 国際語としての英語。未来を担う薬剤師は会話の壁をどう乗り越えるか?, Be Ambitious ! 連載第15回, TURNUP 66,15, Dec.2023
- 橋田亨: 輝ける薬剤師の未来、目に見えない壁を超えて, Be Ambitious ! 最終回, TURNUP 67,15, Feb.2024
- 田村亮: 院内製剤としての解毒・拮抗薬の実際;調製側の立場から. 中毒研究, 36(4): 341-346, 2023
- 宗村雅男: 未承認薬・適応外薬に対する行政の取り組みと中毒治療領域の現状. 中毒研究, 36(4): 347-354, 2023
- 薩摩由香里: 振り返れば国試. 第7回高齢者への服薬指導で薬剤師がアセスメントすべきポイントは? 月刊薬事 66(1): 110-116, 2024
2022年度 誌上発表
著書
- 高瀬友貴,室井延之:自律搬送ロボットHOSPi®︎の導入-積極的な活用で医療の発展向上につなげる.医事業務 産労総合研究所.産労総合研究所,東京,29:14-16,2022
- 高瀬友貴:入院患者の栄養療法.月刊薬事臨時増刊号,入江利行(編集),じほう,東京,64:62-72,2022
- 増本憲生:2型糖尿病.薬剤師のための薬物療法問題集.家入一郎(監修),じほう,東京,100-105,2022
- 入江 慶:抗アンドロゲン薬.臨床腫瘍薬学,日本臨床腫瘍薬学会(編集),第2版,じほう,東京,251-254,2022
- 池末裕明:免疫関連有害事象(irAE).臨床腫瘍薬学,日本臨床腫瘍薬学会(編集),第2版,じほう,東京,769-776,2022
- 池末裕明,橋田 亨:抗悪性腫瘍薬.薬剤リスト編.治療薬ハンドブック2023,堀 正二,菅野健太郎,門脇 孝,乾 賢一,林 昌洋(編集),じほう,東京,1046-1173,2023
- 池末裕明:処方箋に基づく医薬品の調製(4)ケミカルハザード.薬学生のための病院・薬局実務実習テキスト2023年版,薬学教育協議会 病院・薬局実務実習近畿地区調整機構(監修),日本病院薬剤師会近畿ブロック/日本薬剤師会大阪・近畿ブロック(編集),じほう,東京,98-199,2023
- 池末裕明:肺がん.がん化学療法レジメン管理マニュアル,濱 敏弘(監修),青山 剛,池末裕明,内田まやこ,佐藤淳也,高田慎也,土屋雅美(編集),第4版,医学書院,東京,183,2023
- 池末裕明:頭頸部がん.がん化学療法レジメン管理マニュアル,濱 敏弘(監修),青山 剛,池末裕明,内田まやこ,佐藤淳也,高田慎也,土屋雅美(編集),第4版,医学書院,東京,733,2023
- 𠮷野新太郎:肺がん(29) ゲフィチニブ (30) エルロチニブ (31) アファチニブ (32) オシメルチニブ.がん化学療法レジメン管理マニュアル,濱 敏弘(監修),青山 剛,池末裕明,内田まやこ,佐藤淳也,高田慎也,土屋雅美(編集),第4版,医学書院,東京,222-236,2023
論文
- Hirabatake M, Mizuno T, Kato K, Hashida T: Everolimus pharmacokinetics and exposure-response relationship in Japanese patients with advanced breast cancer. Front Pharmacol. 13: 984002, 2022
- Hirabatake M, Ikesue H, Iwama Y, Irie K, Yoshino S, Yamasaki T, Hashida T, Kawakita M, Muroi N: Pharmacist–urologist collaborative management improves clinical outcomes in patients with castration-resistant prostate cancer receiving enzalutamide. Front Pharmacol. 13: 901099, 2022
- Takase T, Tsugawa N, Sugiyama T, Ikesue H, Eto M, Hashida T, Tomii K, Muroi N: Association between 25-hydroxyvitamin D levels and COVID-19 severity in Japanese patients. Clinical Nutrition ESPEN. 49: 256-263, 2022
- Takase T, Masumoto N, Shibatani N, Matsuoka Y, Tanaka F, Hirabatake M, Kashiwagi H, Nishioka I, Ikesue H, Hashida T, Koide N, Muroi N: Evaluating the safety and efficiency of robotic dispensing systems. J Pharm Health Care Sci. 8: 24, 2022
- Yamaoka K, Fujiwara M, Uchida M, Uesawa Y, Muroi N, Shimizu T: Comprehensive analysis of ixazomib-induced adverse events using the Japanese pharmacovigilance database. Oncology. 100: 413-418, 2022
- Yamaoka K, Fujiwara M, Uchida M, Uesawa Y, Muroi N, Shimizu T: Comprehensive analysis of adverse events induced by PARP inhibitors using JADER and time to onset. Life. 12: 1355, 2022
- Hiya S, Fujiwara S, Tanaka F, Kohara N, Kawamoto M: High-dose immunoglobulin-dependent chronic demyelinating inflammatory polyneuropathy successfully managed with subcutaneous immunoglobulin using pharmacokinetic analysis. eNeurological Sci. 27: 100404, 2022
- Hasegawa S, Ikesue H, Satake R, Inoue M, Yoshida Y, Tanaka M, Matsumoto K, Wakabayashi W, Oura K, Muroi N, Hashida T, Iguchi K, Nakamura M: Osteonecrosis of the jaw caused by denosumab in treatment-naïve and pre-treatment with zoledronic acid groups: a time-to-onset study using the Japanese Adverse Drug Event Report (JADER) database. Drugs – Real World Outcomes. 9: 659-665, 2022
- Irie K, Hiramoto N, Ishikawa T, Fukushima S: Use of liquid chromatography-tandem mass spectrometry for foscarnet quantification in human serum and cerebrospinal fluid. Rapid Commun Mass Spectrom. 36: e9255, 2022
- Nishioka H, Cho Y, Irie K, Kanamori M: Ceftriaxone-associated encephalopathy in a patient with high levels of ceftriaxone in blood and cerebrospinal fluid. Int J Infect Dis. 116: 223-225, 2022
- Maeda A, Ando H, Irie K, Hashimoto N, Morishige JI, Fukushima S, Okada A, Ebi H, Matsuzaki M, Iwata H, Sawaki M: Effects of ABCB1 and ABCG2 polymorphisms on the pharmacokinetics of abemaciclib. Eur J Clin Pharmacol. 78: 1239-1247, 2022
- Minamii T, Shimizu H, Irie K, Nishioka H: Encephalopathy induced by high levels of ceftriaxone in the blood and cerebrospinal fluid. IDCases. 29: e01581, 2022
- Tohi M, Irie K, Mizuno T, Okuyoshi H, Hirabatake M, Ikesue H, Muroi N, Eto M, Fukushima S, Tomii K, Hashida T: Population pharmacokinetics of nivolumab in Japanese real-world patients with non-small cell lung cancer. Ther Drug Monitor. 45: 110-116, 2023
- Maeda A, Ando H, Irie K, Hashimoto N, Morishige JI, Fukushima S, Okada A, Ebi H, Matsuzaki M, Iwata H, Sawaki M: Effects of ABCB1 and ABCG2 polymorphisms on the pharmacokinetics of abemaciclib metabolites (M2, M20, M18). Anticancer Res. 43: 1283-1289, 2023
- Yamaoka K, Fujiwara M, Uchida M, Uesawa Y, Shimizu T: The influence of the rapid increase in the number of adverse event reports for COVID-19 vaccine on the disproportionality analysis using JADER. In Vivo. 37: 345-356, 2023
- 辻本 勉,波多江 崇,奥貞 智,冨田里佳,六車龍介,黒田泰司,恒吉慶子,佐竹正子,増本憲生:病院薬剤師および薬局薬剤師を対象としたインスリン皮下硬結の理解度と指導実態に関する調査.くすりと糖尿病 11:61-71,2022
総説
- 高瀬友貴,津川尚子,杉山峰是,池末裕明,江藤正明,橋田 亨,富井啓介,室井延之:血中25-ヒドロキシビタミンD濃度とCOVID‑19重症化の関連.ビタミン 96:471-473,2022
- 田村 亮,吉廣尚大:血液浄化療法施行時の薬物投与.救急・集中治療 34:1560-1567,2022
- 田村 亮:ABCDアプローチで見極める薬の使い方 心肺停止.月刊薬事 64:55-62,2022
- 田村 亮:集中治療 重症低酸素血症を伴う成人COVID-19患者の生命維持装置非使用下における生存期間への効果(デキサメタゾン12 mg vs. 6 mgの比較).月刊薬事 64:199-200,2022
- 橋田 亨,木原康樹:国家戦略特区の経験から考える最先端医療の費用負担の在り方.病院 81:584-587,2022
- 池村 舞,橋田 亨:医療現場で活躍する薬剤師になるための研究活動のすすめ.薬学教育 6:2022-022,2022
- 池末裕明,室井延之:薬剤師との協働によるがん薬物療法の安全性確保.臨牀と研究 99:1011-1015,2022
- 入江 慶:COVID-19患者における抗ウイルス薬の薬物動態研究.YAKUGAKU ZASSHI 143:239-241,2023
2021年度 誌上発表
著書
- 橋田亨(監), 室井延之(編). 月間薬事臨時増刊号. 1年目薬剤師の強化書. 2021.
- 室井延之. 静脈栄養剤の種類と組成,特徴. 日本臨床栄養代謝学会 JSPENテキストブック2021.288-294, 2021
- 池末裕明, 橋田亨. 下剤(便秘薬). 「新・違いが分かる!同種・同効薬」. 121-127, 2021
- 池末裕明, 橋田亨. 薬剤師の役割. 希少がん・難治がん診療ハンドブック. 205-208, 2021
- 室井延之, 橋田亨. レジデント制度による薬剤師の育成. Pharma-Lead Next. 2: 2-5, 2022
- 奥貞智. キホンの薬物療法①(経口薬・インクレチン関連薬). 糖尿病ケア+. 19: 79-84, 2022
- 池末裕明. 処方箋に基づく医薬品の調製. (4)ケミカルハザード. 薬学生のための病院・薬局実務実習テキスト2022年版. 96-94, 2022
論文
- Miura R, Hirabatake M, Irie K, Ikesue H, Muroi N, Kawakita M, Hashida T. Safety evaluation of enzalutamide dose-escalation strategy in patients with castration-resistant prostate cancer. Urol Oncol. 39: 233.e15-233.e20, 2021
- Ikesue H, Mouri M, Tomita H, Hirabatake M, Ikemura M, Muroi N, Yamamoto S, Takenobu T, Tomii K, Kawakita M, Katoh H, Ishikawa T, Yasui H, Hashida T. Associated characteristics and treatment outcomes of medication-related osteonecrosis of the jaw in patients receiving denosumab or zoledronic acid for bone metastases. Support Care Cancer. 29: 4763-4772, 2021
- Tanaka F, Shibatani N, Fujita K, Ikesue H, Yoshimizu S, Muroi N, Kurimoto Y and Hashida T. Polypharmacy-associated potential contraindications of drug prescriptions in patients with primary angle closure disease in a real-world setting. J Pharm Health Care Sci. 7: 17, 2021
- Yamashita K, Shimomura Y, Ikesue H, Muroi N, Yoshimoto A, Hashida T. Safety and efficacy evaluation of low-dose trimethoprim-sulfamethoxazole for prophylaxis of Pneumocystis pneumonia in HIV uninfected patients undergoing hemodialysis: a retrospective observational study. BMC Infect Dis. 21: 664, 2021
- Irie K, Nakagawa A, Fujita H, Tamura R, Eto M, Ikesue H, Muroi N, Fukushimab S, Tomii K, Hashida T. Population pharmacokinetics of favipiravir in patients with COVID-19. CPT Pharmacometrics Syst Pharmacol. 10: 1161-1170, 2021
- Inoue M, Matsumoto K, Tanaka M, Yoshida Y, Satake R, Goto F, Shimada K, Mukai R, Hasegawa S, Suzuki T, Ikesue H, Liao J, Hashida T, Nakamura M. Analysis of chemotherapy-induced peripheral neuropathy using the Japanese Adverse Drug Event Report database. Sci Rep. 11: 11324, 2021
- Ikesue H, Doi K, Morimoto M, Hirabatake M, Muroi N, Yamamoto S, Takenobu T, Hashida T. Switching from zoledronic acid to denosumab increases the risk for developing medication-related osteonecrosis of the jaw in patients with bone metastases. Cancer Chemother Pharmacol. 87: 871-877, 2021
- Satake R, Matsumoto K, Tanaka M, Mukai R, Shimada K, Yoshida Y, Inoue M, Hasegawa S, Iguchi K, Ikesue H, Shimizu S, Nishida S, Suzuki A, Hashida T, Nakamura M. Analysis of drug-induced gastrointestinal obstruction and perforation using the Japanese Adverse Drug Event Report Database. Front Pharmacol. 12: 692292, 2021
- Ogata M, Ama Y, Ogata T, Hirabatake M, Yasui H, Satake H. Direct oral anticoagulants for the treatment of venous thromboembolism in patients with active cancer. In Vivo. 35: 2747-2753, 2021
- Ikesue H, Doi K, Morimoto M, Hirabatake M, Muroi N, Yamamoto S, Takenobu T, Hashida T. Risk evaluation of denosumab and zoledronic acid for medication-related osteonecrosis of the jaw in patients with bone metastases: a propensity score-matched analysis. Support Care Cancer. 30: 2341-2348, 2021
- Ikesue H, Yamamoto H, Hirabatake M, Hashida T, Chung H, Inokuma T, Muroi N. Risk factors of proteinuria in patients with hepatocellular carcinoma receiving lenvatinib. Biol Pharm Bull. 45: 333-338, 2022
- Ikesue H, Yamaoka K, Matsumoto A, Hirabatake M, Muroi N, Yamasaki T, Kawakita M, and Hashida T. Risk factors of proteinuria and potentially protective effect of renin-angiotensin system inhibitors in patients with renal cell carcinoma receiving axitinib. Cancer Chemother Pharmacol. Online ahead of print
- 倉本恵里子, 室井延之, 平畠正樹, 平山晴奈, 柴谷直樹, 𡈽井朝子, 栗本康夫, 橋田亨. 眼科日帰り手術クリニカルパスにおけるセフカペンピボキシル錠の術後予防抗菌薬としての適否について. 日本化学療法学会雑誌. 69: 249-254, 2021
- 田中智也, 蔵田靖子, 髙瀬尚武, 樋本繭子, 新免徹, 檀和貴, 鍛治園誠, 正岡康幸, 中本秋彦, 名和秀起, 北村佳久, 池末裕明, 室井延之, 千堂年昭, 三木育子. ラムシルマブ投与患者における蛋白尿発現のリスク因子に関する検討. 医療薬学. 47: 250-255, 2021
- 松石邦隆, 福島春子, 大谷恭平, 宮井宏之, 鶴谷茂, 川村修司, 高橋年道, 桑田美子. 新型コロナウイルス感染症病棟を有する総合病院精神神経科の診療現場におけるコロナ禍の影響と対策. 老年精神医学雑誌. 32: 829-836, 2021
- 濱宏仁, 平畠正樹, 池末裕明, 室井延之, 橋田亨. Drug Vial Optimization導入のための使用期限設定に関する考察. 癌と化学療法. 48: 1139-1143, 2021
- 木下恵,柴谷直樹,宮坂萌菜,大江泰,平野達也,入江慶,吉水聡,藤原雅史,栗本康夫,室井延之. 緑内障患者に対する「緑内障薬剤師外来」の評価. あたらしい眼科. 38: 1109-1113, 2021
総説
- 室井延之. 臨床薬剤師の育成につなげる病院実務実習とは. 薬学教育. 5: 2020-2024, 2021
- 室井延之. ロービジョン患者に対する地域連携:病院から地域につなぐロービジョンケアと薬剤師の役割. 薬局. 72: 2489-2493, 2021
- 大江泰. ロービジョン患者から見える薬剤管理. 薬局. 72: 2453-2457, 2021
- 田中郁壮. 緑内障に注意を要する医薬品と代替薬の提案. 薬局. 72: 2458-2464, 2021.
- 宮坂萌菜. 点眼剤の基礎知識. 薬局. 72: 2466-2472, 2021
- 柴谷直樹. ロービジョン患者支援のための点眼剤デバイス開発. 薬局. 72: 2473-2478, 2021
- 平野達也. 緑内障薬剤師外来の開設および取り組み. 薬局. 72: 2495-2505, 2021
- 山田清文, 橋田亨. 薬剤師卒後研修の方向性. 日本病院薬剤師会雑誌. 57: 1190-1196, 2021
- 池末裕明 . 抗がん薬の副作用モニタリングと支持療法. 調剤と情報. 27: 1999-2004, 2021
- 池末裕明. がん薬物療法の継続が困難になるさまざまなシーン. 薬局. 72: 3326-3329, 2021
- 藤田拓俊. 手指消毒、物品消毒. 調剤と情報. 27: 2678-2683, 2021
- 田村亮. 自宅療養患者への往診対応. 調剤と情報. 27: 2714-2718, 2021
- 室井延之, 安田理恵. 地域でのCOVID-19ワクチンの集団接種への対応. 調剤と情報. 27: 2719-2723, 2021
2020年度 誌上発表
著書
- 池末裕明, 伊藤善規, 大石了三. がん化学療法ワークシート第5版. じほう, 東京, 2020
- 薩摩由香里,平野達也:CQ6, CQ16: がん疼痛の薬物療法に関するガイドライン2020年版,日本緩和医療学会ガイドライン統括委員会(編集),金原出版,東京,2020
- 神野正敏, 池末裕明(監), 渡邊裕之(著). 根拠にもとづく がん化学療法レジメン作成とマネジメントのてびき. 羊土社, 東京, 2020
- 池末裕明. 薬学教育協議会 病院・薬局実務実習近畿地区調整機構(監修). 薬学生のための病院・薬局実務実習テキスト2021年版. 処方箋に基づく医薬品の調製. (4)ケミカルハザード. 94-96, じほう, 東京, 2021
- 池末裕明. 「病気とくすり2021」抗がん薬. 1480-1495, 南山堂, 東京, 2021
論文
- Takase T, Ikesue H, Nakagawa H, Kinoshita M, Muroi N, Kitai T, Furukawa Y, Hashida T. Effect of the number of dose adjustment factors on bleeding risk in patients receiving 30 mg/day edoxaban. J Clin Pharm Ther. 45: 298–302, 2020
- Ikesue H, Kusuda K, Satsuma Y, Nishiwaki F, Miura R, Masuda Y, Hirabatake M, Muroi N, Fujimoto D, Morimoto T, Tomii K, Hashida T. Evaluation of the usefulness of protocol-based pharmacist-facilitated laboratory monitoring to ensure the safety of immune checkpoint inhibitors in patients with lung cancer. J Clin Pharm Ther. 45: 6, 1288-1294, 2020
- Torii H, Ando M, Tomita H, Kobaru T, Tanaka M, Fujimoto K, Shimizu R, Ikesue H, Okusada S, Hashida T, Kume N. Association of hypnotic drug use with fall incidents in hospitalized elderly patients: a case-crossover study. Biol Pharm Bull. 43: 925-931, 2020
- Ando M, Nishioka H, Nakasako S, Kuramoto E, Ikemura M, Kamei H, Sono Y, Sugioka N, Fukushima S, Hashida T. Observational retrospective single-centre study in Japan to assess the clinical significance of serum daptomycin levels in creatinine phosphokinase elevation. J Clin Pharm Ther. 45: 290-297, 2020
- Irie K, Nakagawa A, Fujita H, Tamura R, Eto M, Ikesue H, Muroi N, Tomii K, Hashida T. Pharmacokinetics of favipiravir in critically ill patients with COVID-19. Clin Transl Sci. 13: 880-885, 2020
- Uchida M, Yamaguchi Y, Hosomi S, Ikesue H, Mori Y, Maegawa N, Takano A, Sato Y, Hosohata K, Muroi N, Tomii K, Hashida T, Nakamura T. Risk factors for febrile neutropenia induced by docetaxel chemotherapy in patients with non-small cell lung cancer. Biol Pharm Bull. 43: 1235-1240, 2020
- Uchida M, Mori Y, Akiba K, Miyasaka M, Hirano T, Ikesue H, Yamaguchi Y, Takano A, Maegawa N, Shimomura Y, Hosohata K, Muroi N, Ishikawa T, Hashida T, Nakamura T. Risk factors for skin toxicities associated with bendamustine-based chemotherapy in patients with non-Hodgkin lymphoma. Biol Pharm Bull. 43: 10, 1577-1582, 2020
- Hasegawa S, Ikesue H, Nakao S, Shimada K, Mukai R, Tanaka M, Matsumoto K, Inoue M, Satake R, Yoshida Y, Goto F, Hashida T, Nakamura M. Analysis of immune-related adverse events caused by immune checkpoint inhibitors using the Japanese Adverse Drug Event Report database. Pharmacoepidemiol Drug Saf. 29: 1279–1294, 2020
- Satsuma Y, Ikesue H, Kusuda K, Maeda M, Muroi N, Mori R, Kogo M, Hirabayashi R, Nagata K, Nakagawa A, Tachikawa R, Tomii K, Hashida T. Effectiveness of pharmacist-physician collaborative management for patients with idiopathic pulmonary fibrosis receiving pirfenidone. Front Pharmacol, 11:529654, 2020
- Matsumoto K, Hasegawa S, Nakao S, Shimada K, Mukai R, Tanaka M, Satake R, Yoshida Y, Goto F, Inoue M, Ikesue H, Iguchi K, Hashida T, Nakamura, M. Adverse event profile of Reye’s syndrome analyzed using the FDA Adverse Event Reporting System and the Japanese Adverse Drug Event Report databases. SAGE Open Medicine, 8: 1–9, 2020
- Yoshida S, Fujimoto A, Fukushima K, Ando M, Irie K, Hirano T, MiyasakaM, Shimomura Y, Ishikawa T, Ikesue H, Muroi N, Hashida T, Sugioka N. Population pharmacokinetics of tacrolimus in umbilical cord blood transplant patients focusing on the variation in red blood cell counts. J Clin Pharm Ther. 46: 190-197, 2021
- 大音三枝子, 薩摩由香里, 梅田節子, 新城拓也, 西本哲郎, 池末裕明, 室井延之, 橋田亨. ヒドロモルフォン注射剤から経口剤への換算についての検討. 日本緩和医療学会誌. 15: 147-151, 2020
- 登佳寿子, 土肥麻貴子, 藤井尚子, 𡈽井朝子, 室井延之, 橋田亨. HIV感染超低体重出生児に対して抗レトロウイルス療法(ART)を行った1症例. 日本エイズ学会誌. 22: 106-110, 2020
- 土肥麻貴子, 西岡弘晶, 茨木まどか, 池末裕明, 安藤基純, 東別府直紀, 室井延之, 橋田亨. 栄養サポートチームによる中心静脈栄養処方の適正化-当院での検討-. JSPEN. 2: 227-223, 2020
総説
- 橋田亨, 大澤孝, 柏倉康治. 薬剤が抱える医療経済への課題と今後の展望. 薬剤学. 80: 229-232, 2020
- 室井延之. 入退院時/周術期などの移行期におけるシームレスな連携―病院から地域につなぐ薬物療法と薬剤師の役割. 調剤と情報. 26: 1204-1211, 2020
- 室井延之. アレルギー性鼻炎の病態と治療薬. 調剤と情報 臨時増刊号. 26: 176-178, 2020
- 登佳寿子, 室井延之. Column 高齢化社会において、循環器薬のポリファーマシーをどのように考えるか. medicina. 58: 140-141, 2020
- 池末裕明. シリーズ抗がん薬治療の副作用 No. 4. 副作用管理:免疫関連有害事象(irAE). 日本臨床腫瘍薬学会雑誌. 17: 11-19, 2020
- 池末裕明. 臨床現場におけるエビデンスの理解と発信に向けた教育の取り組み. 薬学教育. 4: 2020-035, 2020
- 六車龍介, 奥貞智, 冨田里佳, 増本憲生, 黒田泰司, 辻本勉. 兵庫県(糖尿病療養指導士兵庫県連合会)における活動報告. くすりと糖尿病. 9: 201-206, 2020
2019年度 誌上発表
著書
- 山本晴菜:抗HCV薬.医療現場のための薬物相互作用リテラシー,大野能之,樋坂章博(編集),南山堂,東京,184-188,2019
- 三沖大介:ハイリスク薬の指導記録.シンプルでわかりやすい薬歴・指導記録の書き方,寺沢匡史(編著),南山堂,東京,90-101,2019
- 池末裕明:VEGF経路阻害薬(血管新生阻害薬:高分子薬).整理して理解する抗がん薬,濱敏弘(監修),じほう,東京,138-148,2019
- 槇原洋子,池末裕明:Lesson 8 便秘に対応する.基本的知識と症例から学ぶ: がん緩和ケアの薬の使い方,岡本禎晃,荒井幸子(編集),じほう,東京,112-119,2019
- 池末裕明,橋田亨:抗悪性腫瘍薬.治療薬ハンドブック2020,髙久史麿(監修),じほう,東京,1008-1108,2020
- 池末裕明:処方箋に基づく医薬品の調製. (4)ケミカルハザード.薬学生のための病院・薬局実務実習テキスト2020年版,薬学教育協議会. 病院・薬局実務実習近畿地区調整機構(監修),じほう,東京,84-86,2020
論文
- Ando M, Nakasako S, Ariyoshi K, Yamaguchi M, Sakizono K, Minowa K, Fukushima S, Sugioka N, Hashida T: Re-elevation of serum amlodipine level after lipid emulsion therapy in an overdose case. J Clin Pharm Ther. 44: 970-973, 2019
- Takase T, Ikesue H, Nakagawa H, Kinoshita M, Muroi N, Kitai T, Furukawa Y, and Hashida T: Risk factors for major bleeding and clinically relevant non-major bleeding in Japanese patients treated with edoxaban. Biol Pharm Bull. 43: 458-462, 2020
- Nakao S, Hasegawa S, Shimada K, Mukai R, Tanaka M, Matsumoto K, Uranishi H, Masuta M, Ikesue H, Hashida T, Iguchi K, Nakamura M: Evaluation of anti-infective-related Clostridium difficile-associated colitis using the Japanese Adverse Drug Event Report database. Int J Med Sci. 17: 921-930, 2020
- Ando M, Hirabatake M, Yasui H, Fukushima S, Sugioka N, Hashida T. A simplified method for therapeutic drug monitoring of mitotane by gas chromatography-electron ionization-mass spectrometry. Biomed Chromatogr. 34: 3, e4776, 2020.
- 増本憲生,久井裕美子,細見健悟,杉田裕貴,小牧佐知子,稲田智子,大槻裕朗,中川雄介:冠動脈ステント留置を目的とする短期入院糖尿病患者における入院時リラグルチド自己注射導入の有用性に関する検討.くすりと糖尿病. 8:299-306,2019
総説
- 室井延之:糖尿病治療の評価と合併症・副作用マネジメント 糖尿病における食事・運動療法の評価とその対応.薬局. 70:1583-1587,2019
- 室井延之:輸液・栄養療法で用いるディバイスの基礎知識.薬局. 70:1728-1732,2019
- 山本晴菜:病院薬剤師が肝炎Coとして活躍.肝炎医療Coこれだけは~基礎からすべての職種がひと目で分かる強みを活かした活動事例まで~.厚生労働科研究費補助金「肝炎ウイルス検査受検から受診、受療に至る肝炎対策の効果検証と拡充に関する研究」(代表研究者:江口有一郎)2019年度報告書
- 池末裕明:血管新生阻害薬の術前休薬.月刊薬事 臨時増刊号. 61:2627-2628,2019
- 池末裕明:がん治療による口内炎対策.月刊薬事 臨時増刊号. 61:2629-2630,2019
- 池末裕明:G-CSF製剤の投与方法.月刊薬事 臨時増刊号. 61:2631-2632,2019
- 登佳寿子:ACE阻害薬・ARB.HEART nursing. 32:6-12,2019
- 柴谷直樹、室井延之:神戸アイセンター病院薬剤部の紹介とロービジョン患者への服薬指導の取り組み.全国自治体病院協議会雑誌. 58:611-614,2019
2018年度 誌上発表
著書
- 室井延之、樋本繭子、高瀬尚武:チュータ制度の導入による新人教育制度の構築〜前年度の反省点を生かして〜.薬剤部門における階層別人材育成の理論と実践,赤瀬朋秀(編集),薬ゼミファームブック,埼玉,66-67,2018
- 室井延之:医薬品副作用の影響要因.環境因子─栄養状態・食事・嗜好品・ポリファーマシー.医薬品副作用アセスメント,日本医薬品安全性学会(監修),南山堂,東京,40-43,2019
- 室井延之:医薬品副作用の影響要因.全身障害アセスメント.シーン2 オキサリプラチン(白金製剤)によるショックが疑われた!.医薬品副作用アセスメント,日本医薬品安全性学会(監修),南山堂,東京,86-89,2019
- 室井延之:医薬品副作用の影響要因.全身障害アセスメント.シーン4 インフリキシマブ(抗体医薬)によるショックが疑われた!.医薬品副作用アセスメント,日本医薬品安全性学会(監修),南山堂,東京,95-98,2019
- 室井延之:医薬品副作用の影響要因.血液・造血器障害アセスメント.シーン2 フルオロウラシル(抗がん薬)による顆粒球減少が疑われた!.医薬品副作用アセスメント,日本医薬品安全性学会(監修),南山堂,東京,153-156,2019
- 池末裕明:処方箋に基づく医薬品の調製. (3)ケミカルハザード.薬学生のための病院・薬局実務実習テキスト2019年版,薬学教育協議会 病院・薬局実務実習近畿地区調整機構(監修),じほう,東京,82-85,2019
- 池末裕明,橋田 亨:抗悪性腫瘍薬.治療薬ハンドブック2019,髙久史麿(監修),じほう,東京,1008-1108,2019
- 平畠正樹:静脈血栓塞栓症.臨床腫瘍薬学,臨床腫瘍薬学会(編集),じほう,東京,657-663,2019
- 池末裕明:免疫関連有害事象(irAE).臨床腫瘍薬学,臨床腫瘍薬学会(編集),じほう,東京,769-776,2019
論文
- Takase T, Ikesue H, Tohi M, Ueta H, Mima H, Koyama T, Hashida T: Interaction between warfarin and short-term intravenous amiodarone in intensive care unit patients after cardiac surgery. J Pharm Health Care Sci. 4: 13, 2018
- Yamamoto H, Ikesue H, Ikemura M, Miura R, Fujita K, Chung H, Suginoshita Y, Inokuma T, Hashida T: Evaluation of pharmaceutical intervention in direct-acting antiviral agents for hepatitis C virus infected patients in an ambulatory setting: a retrospective analysis. J Pharm Health Care Sci. 4: 17, 2018
- Ikesue H, Nagano T, Hashida T: A case of acute kidney injury associated with dabrafenib and trametinib treatment for metastatic melanoma. Ann Pharmacother. 52:1051-1052, 2018
- Irie K, Shobu S, Hiratsuji S, Yamasaki Y, Nanjo S, Kokan C, Hata A, Kaji R, Masago K, Fujita S, Okada Y, Katakami N, Fukushima S: Development and validation of a method for gefitinib quantification in dried blood spots using liquid chromatography-tandem mass spectrometry: Application to finger-prick clinical blood samples of patients with non-small cell lung cancer. J Chromatogr B Analyt Technol Biomed Life Sci. 1087-1088: 1-5, 2018
- Irie K, Okada A, Yamasaki Y, Kokan C, Hata A, Kaji R, Fukushima K, Sugioka N, Okada Y, Katakami N, Fukushima S: An LC-MS/MS method for absolute quantification of nivolumab in human plasma: application to clinical therapeutic drug monitoring. Ther Drug Monit. 40: 716-724, 2018.
- Okada A, Kariya M, Irie K, Okada Y, Hiramoto N, Hashimoto H, Kajioka R, Maruyama C, Kasai H, Hamori M, Nishimura A, Shibata N, Fukushima K, Sugioka N: Population pharmacokinetics of vancomycin in patients undergoing allogeneic hematopoietic stem-cell transplantation. J Clin Pharmacol. 58: 1140-1149, 2018
- Masago K, Irie K, Fujita S, Imamichi F, Okada Y, Katakami N, Fukushima S, Yatabe Y: Relationship between paronychia and drug concentrations of epidermal growth factor receptor tyrosine kinase inhibitors. Oncology. 95: 251-256, 2018
- Oshima E, Mibu M, Kubota M, Hashida T: Use of supplements by Japanese cancer patients receiving outpatient cancer chemotherapy. J Altern Complement Med. 24: 1003-1006, 2018
- Torii H, Shimizu R, Tanizaki Y, Omiya Y, Yamamoto M, Kamiike S, Yasuda D, Hiraoka Y, Hashida T, Kume N: Effects of ramelteon and other sleep-promoting drugs on serum low-density lipoprotein and non-high-density lipoprotein cholesterol: A retrospective comparative pilot study. Biol Pharm Bull. 41: 1778-1790, 2018
- Fujimoto K, Iwakura T, Aburaya M, Matsuoka N: Twice‑daily insulin degludec/insulin aspart effectively improved morning and evening glucose levels and quality of life in patients previously treated with premixed insulin: an observational study. Diabetology & Metabolic Syndrome. 10: 64, 2018
- Kotake T, Satake H, Okita Y, Hatachi Y, Hamada M, Omiya M, Yasui H, Hashida T, Kaihara S, Inokuma T, Tsuji A: Prevalence and risk factors of hepatitis B virus reactivation in patients with solid tumors with resolved HBV infection. Asia Pac J Clin Oncol. 15: 63-68, 2018
- Hirabayashi R, Fujimoto D, Satsuma Y, Hirabatake M, Tomii K: Successful oral desensitization with osimertinib following osimertinib-induced fever and hepatotoxicity: a case report. Invest New Drugs 36: 952-954, 2018
- Maeda A, Irie K, Ando H, Hasegawa A, Taniguchi H, Kadowaki S, Muro K, Tajika M, Aoki M, Inaguma K, Kajita M, Fujimura A, Fukushima S: Associations among regorafenib concentrations, severe adverse reactions, and ABCG2 and OATP1B1 polymorphisms. Cancer Chemother Pharmacol. 83: 107-113, 2019
- Irie K, Okada A, Masuda Y, Fukushima K, Sugioka N, Okuda C, Hata A, Kaji R, Okada Y, Katakami N, Fukushima S: Assessment of exposure risk of irinotecan and its active metabolite, SN-38, through perspiration during chemotherapy. J Oncol Pharm Pract. 25: 865-868, 2019
- 倉本恵里子,平畠正樹,中浴伸二,安藤基純,池末裕明,平井雄三,𡈽井朝子,橋田亨:歯科領域における日帰り手術後の経口抗菌薬適正使用に向けた取り組みとその効果.医療薬学. 44:422-428,2018
- 高瀬友貴,池末裕明,片岡美咲,尾山将樹,三沖大介,藤井尚子,奥貞 智,室井延之,橋田亨:院外処方せんの疑義照会に薬剤師が回答する院内プロトコールの導入とその効果.医療薬学. 45:82-87,2019
- 中川幸紀,三木育子,髙瀬尚武,田中智也,三浦紋佳,濱口常男,高尾雄二郎,勝谷誠,三井康裕,室井延之:直接作用型抗ウイルス薬(Direct acting antivirals: DAAs)の副作用発現状況ならびに危険因子に関する検討.日病薬雑誌. 55:177-182,2019
総説
- 橋田亨:医療現場で活躍する実力派薬剤師の養成は最初の一歩が肝心.薬学教育. 2:113-116,2018
- 池末裕明:入院から周術期、退院後までをつなぐ薬物療法マネジメント.HosPha. 29:4-8,2019
- 池末裕明:癌治療補助薬.医薬ジャーナル増刊号. 新薬展望2019. 55:S1:169-172,2019
2017年度 誌上発表
著書
- 橋田亨,西岡弘晶(編集)
- 薬剤師レジデントマニュアル 第2版.医学書院,東京,2018
- 池末裕明(分担執筆). 吉村知哲,田村和夫(監修) 浮腫.がん薬物療法副作用管理マニュアル, 医学書院,東京,103-9, 2018
- 池末裕明,平畠正樹(分担執筆). 日本医療薬学会(編集) 前立腺がん.病態を理解して組み立てる. 薬剤師のための疾患別薬物療法Ⅰ. 悪性腫瘍, 南江堂,東京,46-59, 2018
- 池末裕明(分担執筆) 抗悪性腫瘍薬.薬局増刊号.病気とくすり2018, 69:1438-1452,南山堂,東京,2018
- 池末裕明(分担執筆). 安藤雄一,寺田智祐(編集).南博信(監修) 特殊な臨床背景でのがん薬物療法:PS不良例.ハイリスク患者のがん薬物療法ハンドブック, 羊土社,東京,296-301, 2017
論文
- Ikemura M, Hashida T: Effect of hyperglycemia on antitumor activity and survival in tumor-bearing mice receiving oxaliplatin and fluorouracil. Anticancer Res 37: 5463-8, 2017
- Nanjo S, Hata A, Okuda C, Kaji R, Okada H, Tamura D, Irie K, Okada H, Fukushima S, Katakami N: Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer 118: 32-7, 2018
- Uchida M, Nakamura T, Makihara Y, Suetsugu K, Ikesue H, Mori Y, Kato K, Shiratsuchi M, Hosohata K, Miyamoto T, Akashi K: Comparison of antiemetic effects of granisetron and palonosetron in patients receiving bendamustine-based chemotherapy. Pharmazie 73: 304-8, 2018
- 楠田かおり, 西岡和子, 池村舞, 東別府直紀, 西岡晶弘, 橋田亨: ペクチン含有濃厚流動食の下痢改善効果に対する胃酸分泌抑制薬の影響. 日本静脈経腸栄養学会雑誌 32: 988-91, 2017
- 池村舞, 安藤基純, 橋田亨: 薬剤師が取り組む臨床研究への支援方法の提案. 薬学教育 1: 2017
- 畑中由香子, 三浦理恵子, 永田一真, 奥貞智, 池末裕明, 藤本大智, 岩本善嵩, 中川淳, 上野聖子, 三石哲也, 奥新浩晃, 富井啓介, 橋田亨: フェブキソスタットとアザチオプリンの相互作用による汎血球減少症の1症例. 日本病院薬剤師会雑誌 54: 53-6, 2018
- 高栁信子, 奥野昌宏, 久保嘉靖, 橋本朗子, 田中康博, 高蓋寿朗, 新里偉咲, 池末裕明, 中田学: 医師の診察に同席した薬剤師からの処方提案がレナリドミド治療に与える影響.日本病院薬剤師会雑誌 54: 167-74, 2018
総説
- 奥貞智: 患者さんのお薬に対する疑問に答えますお薬Q&A ①インスリン注射. 月刊糖尿病ライフさかえ 58: 23, 2018
- 森本茂文: 今期のケモリーグを大予想. がん化学療法おくすり選手名鑑.プロフェッショナルがんナーシング 7: 14-5, 2017
- 森本茂文: 今期のケモリーグを大予想. 監督が語る、がん種別チーム戦略! がん化学療法おくすり選手名鑑. プロフェッショナルがんナーシング 7: 45-7, 2017
2016年度 誌上発表
著書
- 青山剛, 東加奈子, 池末裕明, 川上和宜, 佐藤淳也, 橋本浩伸(編集). 濱敏弘(監修)
がん化学療法レジメン管理マニュアル 第2版.医学書院,東京,2016 - 池末裕明(分担執筆). 古川 裕之(編集)
悪性新生物の基礎と治療薬. 抗がん薬. - 病気とくすり2016, 薬局増刊号, 南山堂,東京,1342-55, 2016
論文
- Rumiko Shimizu, Haruki Torii, Daisuke Yasuda, Yoshinori Hiraoka, Yutaka Furukawa, Akihiro Yoshimoto, Toshio Iwakura, Naoki Matsuoka, Keisuke Tomii, Nobuo Kohara, Tohru Hashida, Noriaki Kume: Comparison of serum lipid management between elderly and non-elderly patients with and without coronary heart disease (CHD). Prev Med Rep 4: 192-8, 2016
- Kimitaka Suetsugu, Hiroaki Ikesue, Toshihiro Miyamoto, Motoaki Shiratsuchi, Nanae Yamamoto-Taguchi, Yuichi Tsuchiya, Kumi Matsukawa, Mayako Uchida, Hiroyuki Watanabe, Koichi Akashi, Satohiro Masuda: Analysis of the variable factors influencing tacrolimus blood concentration during the switch from continuous intravenous infusion to oral administration after allogeneic hematopoietic stem cell transplantation. Int J Hematol 105: 361-8, 2017
- 金光佳織,森本茂文,北田徳昭,片岡和三郎,青木卓哉,吉岡信也,北正人,橋田亨: 妊婦の風疹抗体価に基づく風疹ワクチン接種推奨の必要性に関する検討. 医療薬学 42: 687-93, 2016
- 杉山有吏子, 池村舞, 奥貞智, 岩倉敏夫, 橋田亨: 1型糖尿病患者における既存持効型インスリンからインスリンデグルデクへの変更による血糖コントロールの評価. 医療薬学 42: 562-8, 2016
- 石田茂, 武田真樹, 尾川理恵, 中島貴史, 池末裕明, 渡邊裕之, 金谷朗子, 江頭伸昭, 増田智先: 集中治療室における注射剤配合変化早見表の作成と有用性の評価. 医療薬学 42: 286-94, 2016
- 鹿子木成美, 末次王卓, 高田敦史, 池末裕明, 渡邊裕之, 福田未音, 了戒百合子, 金谷朗子, 江頭伸昭, 増田智先: IT支援システムの構築による退院時薬剤情報管理指導の効率化. 日本病院薬剤師会雑誌 52: 882-6, 2016
総説
- 池末裕明, 秦晃二郎, 了戒百合子, 大畠俊一, 末次王卓, 渡邊裕之, 増田智先: がん骨転移治療薬デノスマブによる低カルシウム血症の発現時期と危険因子. 臨床薬理の進歩 142-8, 2016
2015年度 誌上発表
著書
- 橋田 亨, 西岡弘晶(編集). 薬剤師レジデントの鉄則, 医学書院,東京,2016
論文
- Mai Ikemura, Shinji Nakasako, Ryutaro Seo, Takahiro Atsumi, Koichi Ariyoshi, Tohru Hashida: Reduction in gastrointestinal bleeding by development and implementation of a protocol for stress ulcer prophylaxis:a before-after study. J Pharm Health Care Sci 1: 33, 2015
- Rumiko Shimizu, Haruki Torii, Daisuke Yasuda, Yoshinori Hiraoka, Noriaki Kitada, Tohru Hashida, Akihiro Yoshimoto, Toru Kita, Noriaki Kume: Serum Lipid Goal Attainment in Chronic Kidney Disease (CKD) Patients under the Japan Atherosclerosis Society (JAS) 2012 Guidelines. A Single-Center Study. J Pharm Health Care Sci 22: 949-57, 2015
- 濱 宏仁, 田中詳二, 橋田 亨: オゾン水おび次亜塩素酸ナトリウムを用いた抗がん薬汚染環境の除染効果. 医療薬学 41: 740-49, 2015
- 池村 舞, 橋田 亨: Pharmacist-Scientistsの育成を目的とした修了課程に基づく研究経験の評価. YAKUGAKU ZASSHI 135: 131-7, 2016
総説
- 大音三枝子 せん妄特集PART-1 なぜ?せん妄ケアが重要なワケ. ナース専科 1: 16-9,2016
- 大音三枝子 せん妄特集PART-3 リスク因子からせん妄を見極めよう. ナース専科 1: 33-4,2016
2015年度 学会発表・講演
- Hashida T:Special Lecture II The New Role Required for Pharmacists in Cancer Pharmacotherapy. The 13th China-Japan-Korea (CJK) Joint Symposium for Clinical Information on Parenteral Drugs in Kyoto,2015.4.18
- Motozumi Ando, Eriko Kobiki, Shinji Nakasako, Hiroko Kashiwagi, Noriaki Kitada, Shigefumi Morimoto, Hiroki Kamei, Yumi Sono, Hiroaki Nishioka, Tsuyoshi Fukuda, Tohru Hashida: Safety and pharmacokinetics of daptomycin in Japanese patients, IATDMCT 2015, Rotterdam,2015.10.11-15
- 橋田亨:がん患者を対象とした薬剤師外来~がん患者指導管理料算定のコツも含めて~,Nagoya Clinical Pharma Symposium,名古屋,2015.21
- 杉山有吏子, 池村 舞, 奥貞 智, 岩倉敏夫, 橋田 亨:1型糖尿病患者における既存持効型インスリン製剤1日2回投与からデグルデク1日1回投与への切り替えに関する検討. 第58回日本糖尿病学会年次学術集会, 下関, 2015.5.21-24
- 山本千代:CML治療における服薬アドヒアランス向上を目指して~当院薬剤師外来の現状~. 第30回兵庫県病院薬剤師のためのオンコロジーセミナー, 神戸, 2015.5.28
- 橋田 亨:これからの医療を担う薬剤師への期待,平成27年全国済生会病院薬剤師会研修会,大阪,2015.5.30
- 橋田 亨:がん薬物治療におけるがん専門薬剤師の役割,大阪薬科大学 第8回公開シンポジウム,高槻,2015.6.5
- 稲角利彦:急性期病院の緩和ケアチームにおける薬剤師の役割. 第5回大学医療連携講演会, 神戸, 2015.6.10
- 橋田亨:薬剤師レジデントから始める薬剤師のキャリアパス,日本医療マネジメント学会学術総会,大阪,2015.6.13
- 山本晴菜:C型肝炎に対するダクラタスビル・アスナプレビル経口2剤治療の副作用について. 第30回東神戸消化器疾患セミナー, 神戸, 2015.6.18
- 橋田亨:医療の最前線で活躍する薬剤師を目指して,名城大学薬学部夢・発見セミナー,名古屋,2015.6.20
- 奥貞 智:入院前から退院までシームレスな薬物治療を薬剤師がつなぐ. 全国都市立病院薬局長協議会研修会, 神戸, 2015.7.3
- 橋田亨:抗がん薬のトータルマネジメント〜暴露ゼロとやりがいの職場作り〜,第1回日本医薬品安全性学会,福山,2015.7.4-5
- 宮坂萌菜, 大音三枝子, 森本茂文, 伊藤聡子, 北村昇, 橋田 亨:せん妄チームにおける抑肝散使用患者への薬剤師の介入. 医療薬学フォーラム, 名古屋, 2015.7.4-5
- 柴谷直樹, 小曳恵里子, 奥貞 智, 森本茂文, 橋田 亨:実践的能力を有する薬剤師育成のための屋根瓦方式を活用した実習プログラムの紹介. 医療薬学フォーラム, 名古屋, 2015.7.4-5
- 橋田亨:薬物治療の安全を支える仕組みと人,第40回香川県臨床薬剤師研修会,高松,2015.7.21
- 橋田亨:シームレスな薬物治療を実現する新しい薬剤業務- 薬剤師外来と地域連携 -,平成27年度ヒロシマ薬剤師研修会,広島,2015.7.26
- 森本茂文:業務から考えるクリニカルクエスチョン~臨床研究とデータセンター設立の道程~. 阪神エリアファーマシーセミナー, 西宮, 2015.7.29
- 山本香織:外来治療をサポートする薬剤師の役割~全てはがん患者のために!~. 関西注射剤実践懇話会第25回学術集会, 大阪, 2015.8.8
- 奥貞 智:薬学的管理と薬剤師業務. 神戸市看護大学研修薬剤部紹介, 神戸, 2015.9.9
- 奥貞 智, 冨田里佳, 西濱輝美, 増本憲生, 六車龍介, 辻本 勉:糖尿病療養指導士兵庫県連合会活動の検証〜アンケート調査結果による育成・教育効果〜. 第4回日本くすりと糖尿病学会学術集会, 新潟, 2015.9.26-27
- 中浴伸二:抗微生物薬の効果に影響を与える因子、薬物相互作用、TDMについて. 日本看護協会, 平成27年度感染管理認定看護師教育課程, 神戸, 2015.9.29
- 大音三枝子, 薩摩由香里, 稲角利彦, 梅田節子, 李 美於, 橋田 亨:フェンタニルクエン酸塩舌下錠の安全な使用に向けた当院の取り組みとその有用性について. 第9回緩和医療薬学会, 横浜, 2015.10.2-4
- 佐久間美佐緒, 奥貞 智, 繁平清美, 水本美也子, 冨沢淑子, 瓜生原健嗣:入院前検査センターにおける多職種連携による薬剤リスク回避~周術期における看護師・薬剤師連携による早期介入とその評価~. 第54回全国自治体病院学会, 函館, 2015.10.8-9
- 奥貞 智:糖尿病薬(内服)の種類と使い方~患者指導の方法~. 兵庫県糖尿病療養指導士連合会糖尿病セミナー講習会, 神戸, 2015.10.11-12
- 奥貞 智:糖尿病患者への関わりを再考する. DM FLAG in 兵庫, 神戸, 2015.10.15
- 登 佳寿子:循環器疾患の2次予防を考える~薬剤師の立場から~. 循環器病疾患講演会, 神戸, 2015.10.15
- 中浴伸二:臨床で注意すべき抗菌薬の薬物動態(教育講演). 第58回日本感染症学会中日本地方会学術集会, 奈良, 2015.10.16
- 平畠正樹: 適切な副作用マネジメントによる治療効果の最大化を目指して~各施設における取り組み~. スチバーガWEBカンファレンス, 2015.10.19
- 橋田亨:急性期病院における薬剤部門マネジメント~激変する医療環境に対応する戦略と機能~,第一回熊本市公的病院薬剤部長マネジメントカンファランス,熊本,2015.10.27
- 橋田亨:シンポジウム・就労を支援するがん薬物療法の実際,第53回日本癌治療学会学術集会,京都,2015.10.29-31
- 大音三枝子:せん妄ケアによるインシデントを防ぐための工夫~せん妄チームにおける薬剤師の役割~. 兵庫県看護協会支部共同研修会(神戸中部), 神戸, 2015.11.14
- 池村 舞, 中浴伸二, 藤原秀敏, 土肥麻貴子, 瀬尾龍太郎, 渥美生弘、有吉孝一、橋田 亨:プロトコルに基づいた薬物治療管理の実践によるストレス潰瘍予防効果の改善. 第25回日本医療薬学会, 横浜, 2015.11.21
- 高瀬友貴, 土肥麻貴子, 藤原秀敏, 登佳寿子, 森本茂文, 早坂朋彦, 植田浩司,池村 舞, 橋田 亨:リファンピシンによる代謝酵素誘導に対してワルファリン、ブコローム、アミオダロン併用化にPT-INRをコントロールできた1症例 . 第25回日本医療薬学年会, 2015.11.21-22
- 山本晴菜, 山本香織, 左近絢子, 池村 舞, 鄭 浩柄, 杉之下与志樹, 猪熊哲朗, 橋田 亨:C型肝炎インターフェロンフリー治療中の患者を対象とした薬剤師外来の開設とその評価, 第25回日本医療薬学会年会, 横浜, 2015.11.22
- 橋田 亨:入院前から周術期、転・退院までをつなぐ薬剤業務,第25回日本医療薬学会年会, 横浜, 2015.11.21-23
- 橋田 亨:抗がん剤アドヒアランス向上を目指した薬剤師外来の活用,第25回日本医療薬学会年会, 横浜, 2015.11.21-23
- 橋田 亨:入院・外来から地域へシームレスに薬物療法をつなぐ,京都府薬剤師会舞鶴地区第8回学術講演会,舞鶴,2015.11.25
- 大音三枝子:せん妄チームにおける薬剤師の役割. 大阪薬剤師会せん妄講演会, 大阪2015.11.27
- 山本晴菜:C型肝炎インターフェロンフリー治療に対する 病院薬剤師の取り組みと応需薬局との連携. 兵庫県病院薬剤師会 Hepatitis C Pharmacist Seminar 2015, 神戸, 2015.11.27
- 奥貞 智:糖尿病患者への関わりを再考する. 第2回Awaji Pharmacist Team Conference, 兵庫県淡路, 2015.12.3
- 平畠正樹: がん化学療法におけるCDTM. 第6回大学-医療連携講演会(神戸学院大学),神戸, 2015.12.4
- 橋田亨:広がり高まる病棟薬剤師のニーズに応える,信州病棟薬剤師セミナー,松本,2015.12.11
- 清水里紗, 柴谷直樹, 池村 舞, 奥貞 智, 森本茂文, 橋田 亨:薬学教育新モデル・コアカリキュラムに先行した実習プログラムの実施と課題. 第37回日本病院薬剤師会近畿学術大会, 神戸, 2016.1.23-24
- 佐久間美佐緒, 吉野有香子, 奥貞 智, 池村 舞, 繁平清美, 水本美也子, 冨沢淑子, 森本茂文, 瓜生原健嗣, 橋田 亨:入院前からの常用薬への薬剤師早期介入に対する医師・看護師・薬剤師の評価. 第37回日本病院薬剤師会近畿学術大会, 神戸, 2016.1.23-24
2014年度 誌上発表
著書
- 金光佳織(分担執筆). 古川 裕之(編集)
各論Ⅱ病棟業務がスムーズに進む48のツボ1.持参薬管理 1-3.周術期.病棟薬剤業務ハンドブック, じほう,東京,59-61, 2014 - 北田徳昭(分担執筆). 古川 裕之(編集)
各論Ⅱ病棟業務がスムーズに進む48のツボ5.プロトコル作成.病棟薬剤業務ハンドブック, じほう,東京,86-7, 2014 - 森本茂文(分担執筆). 古川 裕之(編集)
各論Ⅱ病棟業務がスムーズに進む48のツボ6.副作用モニタリング6-2.CTCAEによる評価・記録.病棟薬剤業務ハンドブック, じほう,東京,94-5, 2014 - 中浴伸二(分担執筆). 古川 裕之(編集)
各論Ⅱ病棟業務がスムーズに進む48のツボ7.他職種との連携 7-2.迅速な情報共有、チームワーク形成.病棟薬剤業務ハンドブック, じほう,東京,100-4, 2014
論文
- 森本茂文、宮部貴識、井上綾子、畑裕基、谷島裕之、前田恒宏、庄野嘉治、仲谷芙美、藤田芳一、山崎邦夫、堀内哲也: mFOLFOX6療法による早期血液毒性と栄養評価指数PNIに関する検討.外科と代謝・栄養 48: 59-67, 2014
- 山本晴菜、北田徳昭、柴谷直樹、平畠正樹、橋田亨: 新規医薬品導入時におけるリスク最小化活動のための留意点と薬剤師の役割 デノスマブを例に.医薬品情報学 16: 28-32, 2014
- 田中恒明、竹内昌司、橋田亨、小竹慶子、内川清次、今泉幸久: 病棟薬剤業務を推進することによる薬剤管理指導業務と薬剤関連インシデントへの影響. 日本病院薬剤師会雑誌 50: 1226-9, 2014
総説等
- 櫻井美由紀、阿南節子、石丸博雅、藤井千賀、森本茂文、岩本寿美代、吉田仁: 平成25年度学術委員会学術第7小委員会報告 抗がん薬安全取り扱いに関する指針の作成に向けた調査・研究(最終報告).日本病院薬剤師雑誌 50: 1065-71,2014
- 橋田亨: 薬剤師レジデント制度 わが国の現状と当院での取り組み. 全国自治体病院協議会雑誌 53: 881-6, 2014
- 池村舞、橋田亨: 【神経疾患治療薬の剤形と使い分け】 錠剤と徐放錠の製剤学的特徴. Brain Medical 26: 95-102, 2014
- 橋田亨: 病院のミッションを見据えたファーマシーマネジメント. 薬局 65: 2574-80, 2014
- 橋田亨: 【外科医が知っておきたい医療安全】 医療安全への薬剤師の積極的関与. 消化器外科 37: 1703-10, 2014
- 橋田亨: 【チーム医療における病院薬剤師の役割】 外来チーム医療における病院薬剤師の役割と人材養成. 病院 73: 779-83, 2014
- 橋田亨: 【薬剤師教育の実践的ヒント-薬剤師力を伸ばす!】 レジデントプログラムから学ぶ新人・若手教育 わが国のレジデント制度と神戸市立医療センター中央市民病院のケース. 月間薬事 56: 1969-75, 2014
- 奥貞智, 橋田亨: 周術期患者への薬学的介入(シームレスな関わりを目指す):薬学管理のポイント:術前(外来・入院前).月刊薬事 57: 27-33, 2015
2014年度 学会発表・講演
- 玉木理衣,森亜弥,柳原五吉,天野富美夫:Biology and Pathology of Chemotherapeutic treatment for Gastric scirrhous cancer.Drug Discovery & Therapy World Congress 2014,ボストン(アメリカ),2014.06.18
- 山本千代,入江慶,岡田裕,小野祐一郎,橋本尚子,石川隆之,橋田亨:Effect of Neurokinin-1 Receptor Antagonist on Tacrolimus Concentration in Allogeneic Stem Cell Transplantation.The 3rd International Symposium of Training Plan for Oncology Professionals,大阪,2015.02.07
- 稲角利彦:腰痛により臥位で過ごすことが困難であった原発不明癌の症例.第15回阪神緩和薬物療法ネットワーク,尼崎,2014.04.08
- 奥貞智:糖質制限食に関する医療者側の意識・取り組み状況〜アンケート調査結果から薬剤師の対応を検討〜.第13回糖尿病療養指導士兵庫県連合会講演会,神戸,2014.04.19
- 森本茂文:がん患者指導管理料を算定してわかったことⅠ. 3rdActive Pharmacist Seminar inHYOGO,神戸,2014.05.16
- 平畠正樹,橋田亨:中等度催吐性リスク抗がん薬に対する制吐療法の現状報告と課題.第6回JSOPP,東京,2014.05.18
- 森本茂文,畑裕基,宮部貴識,山﨑邦夫:曝露対策の現状報告と課題.第6回日本がん薬剤学会(JSOPP),東京,2014.05.18
- 平畠正樹:医師と薬剤師の合意に基づく薬物治療管理と処方提案.平成26年度国公私立大学病院医療技術関係職員研修,東京,2014.05.29
- 平畠正樹:がん医療と薬理.がん看護専門研修,神戸,2014.05.29
- 橋田 亨:医療安全への薬剤師の積極的な関与.日本医療安全学会第1回多職種間学際シンポジウム, 大阪 ,2014.05.31
- 橋田亨:新時代薬剤師のミッションと挑戦.千葉県病院薬剤師サミット, 千葉, 2014.6.14
- 森本茂文:私と臨床研究との出会い、続けたい理由.第3回大学-医療連携講演会,神戸,2014.07.04
- 橋田亨:薬剤師外来の構築と現状.愛媛県病院薬剤師会講演会, 松山, 2014.7.5
- 中浴伸二:災害に強い薬剤師になるために -経験に学び、次に備える-. 兵庫県薬剤師会研修部主催講演会,神戸,2014.07.13
- 中浴伸二,安藤基純,園真廉,浅香葉子,井上彰,瀬尾龍太郎,渥美生弘,有吉孝一,北田徳昭,森本茂文,橋田亨:アムロジピン過量内服患者の薬物血中濃度を経時的に測定した1症例.第36回日本中毒学会総会学術集会,東京,2014.07.25
- 森本茂文:副作用マネージメント.肺がんチーム医療推進フォーラム(日本肺がん学会),大阪,2014.07.27
- 谷原明子,藤原歩,奥貞佳奈子,奥貞智,西田明弘,北田徳昭,角本幹夫,小池千恵子,橋田亨:薬剤師による点眼剤の服薬指導状況調査および点眼剤の患者向け説明文書の作成と評価.第5回日本アプライド・セラピューティクス学会学術大会,神戸,2014.08.02
- 玉木理衣:臨床試験における現状と問題点について CRCの立場から.大阪消化管がん化学療法研究会(OGSG)夏季セミナー 消化器がんにおける臨床試験について考える,大阪,2014.08.09
- 八木祐子,平畠正樹,野村洋道,中西真也,北田徳昭,辻晃仁,石川隆之,富井啓介,橋田亨:免疫抑制・化学療法により発症するB型肝炎に対する薬剤部の取り組みとその評価.第52回日本癌治療学会学術集会,横浜,2014.08.30
- 山本香織:S-1とワルファリン併用患者における血液凝固能変動についての実態調査.第52回日本癌治療学会学術集会,横浜,2014.08.30
- 佐藤志保,弓木栞里,柴谷直樹,岸本修一,北田徳昭,西岡和子,青木卓哉,猪爪信夫,橋田亨,福島昭二:切迫早産治療薬リトドリンの血中濃度とSULT1A1およびβ2アドレナリン受容体の遺伝子多型.第64回日本薬学会近畿支部総会大会,京都,2014.09.13
- 玉木理衣,森本茂文,辻晃仁:がん臨床試験とCRC業務の多様化.第15回関西がんチーム医療研究会,大阪,2014.09.13
- 平畠正樹,伊藤浩樹,八木祐子,野村洋道,中西真也,松下章子,橋田亨:がん化学療法終了後患者における免疫抑制・化学療法により発症するB型肝炎対策.第1回日本医療安全学会学術総会,東京,2014.09.21
- 奥貞智:入院前薬剤師外来による常用薬確認の有用性.第1回日本医療安全学会,東京,2014.09.22
- 奥貞智:入院前から退院調整まで薬学的管理をつなぐ.24回日本医療薬学会年会,名古屋,2014.09.27
- 金光佳織,北田徳昭,森本茂文,青木卓哉,北正人,片岡和三郎,橋田亨:妊婦の風疹抗体価に基づく風疹ワクチン接種に関する検討.第24回日本医療薬学会年会,名古屋,2014.09.27
- 八木祐子,平畠正樹,伊藤浩樹,野村洋道,中西真也,松下章子,橋田亨:造血幹細胞移植後患者における免疫抑制・化学療法により発症するB型肝炎対策.第24回日本医療薬学会年会,名古屋,2014.09.27
- 平畠正樹,森本茂文,山本晴菜,北田徳昭,橋田亨:薬剤師外来の経験を活かしたがん患者指導管理業務の要件整備と課題.第24回日本医療薬学会年会,名古屋,2014.09.27
- 登佳寿子,橋田亨:薬物療法専門薬剤師による薬物治療介入の実際と症例サマリーの纏め方(循環器領域).第24回日本医療薬学会年会,名古屋,2014.09.27
- 安藤基純,小曳恵里子,中浴伸二,柏木裕子,北田徳昭,亀井博紀,園諭美,西岡弘晶,福田剛史,橋田亨:抗MRSA薬ダプトマイシンの安全性と血中濃度モニタリングの有用性について.第24回日本医療薬学会年会,名古屋,2014.09. 28
- 中浴伸二:抗微生物薬の効果に影響を与える因子、薬物相互作用、TDMについて.本看護協会 平成26年度感染管理認定看護師教育課程,神戸,2014.09.30
- 稲角利彦,薩摩由香里,左近絢子,金剛圭佑,大音三枝子,北田徳昭,梅田節子,李美於,橋田亨:緩和ケア外来における薬剤師の介入とその評価~第2報~.第8回日本緩和医療薬学会年会,松山,2014.10.04
- 左近絢子,薩摩由香里,大音三枝子,稲角利彦,北田徳昭,梅田節子,李美於,橋田亨:がん患者に対する塩酸デュロキセチンの鎮痛補助効果および安全性の評価.第8回日本緩和医療薬学会年会,松山,2014.10.04
- 奥貞智:糖尿病セミナー講義 糖尿病薬(内服). 糖尿病療養指導士兵庫県連合会「糖尿病セミナー」,神戸,2014.10.12
- 森本茂文:がん患者指導管理料を算定してわかったことⅡ. 第10回関西サイコオンコロジー研究会,大阪,2014.10.31
- 橋田亨:専門性を活かした薬剤業務のパラダイムシフ, 日本医療薬学会第56回公開シンポジウム, 札幌, 2014.11.01
- 奥貞智,冨田里佳,西濱輝美,増本憲生,六車龍介,辻本勉:インスリン注射の早期導入についての意識調査〜調査結果より糖尿病療養指導士の育成への取り組みを検証~.第3回くすりと糖尿病学会学術集会,福岡,2014.11.02
- 杉山有吏子,池村舞,奥貞智,北田徳昭,岩倉敏夫,橋田亨:1型糖尿患者におけるインスリンデグルデクの有効性、安全性に関する検討.第3回くすりと糖尿病学会学術集会,福岡,2014.11.02
- 橋田亨:外来へシフトする薬剤業務のマネジメント. 大阪府下市立病院薬剤部長会学術講演会, 大阪, 2014.11.7
- 橋田亨:がん患者を対象とした薬剤師外来. Kansai Clinical Pharma Symposium, 大阪, 2014.11.08
- 森本茂文:薬学的知見に基づいたがん患者指導管理の実際.兵庫Oncoligy Pharmacy Seminar,神戸,2014.11.12
- 中浴伸二:災害医療における薬剤師の役割.第62回日本職業災害医学会学術大会, 神戸,2014.11.16
- 森本茂文:病棟業務を活かしたがん患者への指導・副作用などアプローチの実際と薬剤師の役割.大阪府病院薬剤師会 平成26年度第2回病棟薬剤業務講習会,大阪,2014.11.18
- 橋田亨:抗がん薬のトータルマネジメント . 和歌山Oncology Pharmacy Seminar, 和歌山, 2014.11.22
- 玉木理衣:これからのクリニカルリサーチへの関わり.大腸癌クリニカルリサーチフォーラム(CCCRF),神戸,2014.11.28
- 登佳寿子:HIV感染症認定薬剤師の活動と薬物療法について.平成26年度兵庫県立病院薬剤師研修会,神戸,2014.11.29
- 油屋恵:糖尿病患者におけるがん化学療法の有効性と安全性の評価~基礎と臨床の連携~.第4回大学-医療連携講演会,神戸,2014.12.01
- 永井美帆:薬物治療の前方、後方連携.大鵬薬品工業 第3回ポートアイランド医看薬薬連携の会,神戸,2014.12.03
- 柴谷直樹,佐藤志保,弓木栞里,鈴木亮佑,岸本修一,北田徳昭,西岡和子,青木卓哉,吉岡信也,猪爪信夫,橋田亨,福島昭二:切迫早産患者におけるリトドリン塩酸塩の効果発現に及ぼすβ2アドレナリン受容体遺伝子多型の影響.第35回日本臨床薬理学会学術大会,松山,2014.12.04
- 奥貞智:兵庫県糖尿病協会栄養部会第70回研修会 講演.兵庫県糖尿病協会栄養部会研修会,神戸,2014.12.06
- 橋田亨:ハイリスク薬の ヒューマンエラーを防ぐ~抗がん剤の場合~. 医薬品安全管理研修会, 東京, 2014.12.6
- 大音三枝子:医療安全研修「せん妄によるインシデントを防ぐための工夫」講演.2014年度神戸中央支部公益社団法人兵庫県看護協会,神戸,2014.12.13
- 平畠正樹:がん化学療法における薬剤師業務の新展開.兵庫県がん診療連携協議会 第7回薬剤師セミナー,神戸,2015.01.17
- 野間千尋,奥貞智,池村舞,佐久間美佐緒,森本茂文,北田徳昭,瓜生原健嗣,橋田亨:周術期における薬物療法への薬剤師の早期介入とその評価~入院前検査センターにおける投薬マネジメント~ 第36回日本病院薬剤師会近畿学術大会,和歌山,2015.01.24
- 奥貞智:入院前から退院後までシームレスに薬物治療をつなぐ.第36回日本病院薬剤師会近畿学術大会,和歌山,2015.01.24
- 稲角利彦:当院におけるアセトアミノフェン注射剤の使用状況.第36回日本病院薬剤師会近畿学術大会,和歌山,2015.01.24
- 笠井祐希,平畠正樹,川口奈奈子,橋田亨:デノスマブ投与患者における腎機能別血清カルシウム値の推移とカルシウム、ビタミンD投与.第36回日本病院薬剤師会近畿学術大会,和歌山,2015.01.25
- 藤原秀敏:地域医療連携から求められる病棟薬剤業務.第36回日本病院薬剤師会近畿学術大会,和歌山,2015.01.25
- 平畠正樹,森本茂文,山本晴菜,北田徳昭,橋田亨:薬剤師外来をベースとしたがん患者指導管理.第36回日本病院薬剤師会近畿学術大会,和歌山,2015.01.25
- 平畠正樹,伊藤浩樹,八木祐子,野村洋道,中西真也,松下章子,橋田亨:免疫抑制・化学療法により発症するB型肝炎対策.近畿ブロックがん専門薬剤師フォーラム,大阪,2015.02.07
- 辰巳智美,池村舞,橋田亨:アルテプラーゼ投与による出血頻度と背景因子についての検討.第4回日本薬剤師レジデントフォーラム,東京,2015.02.07
- 風間正,安藤基純,森本茂文,橋田亨:悪性リンパ腫に対するR-B療法における血液毒性発現の性差に関する検討.第4回日本薬剤師レジデントフォーラム,東京,2015.02.07
- 油屋恵,池村舞,橋田亨:糖尿病患者におけるがん化学療法実施に関する実態調査~有効かつ安全ながん化学療法の確立を目指して~.第4回日本薬剤師レジデントフォーラム,東京,2015.02.07
- 笠井祐希,平畠正樹,川口奈奈子,橋田亨:デノスマブ投与患者における腎機能別血清カルシウム値の推移とカルシウム、ビタミンD投与.第4回日本薬剤師レジデントフォーラム,東京,2015.02.07
- 野間千尋,奥貞智,池村舞,安藤基純,佐久間美佐緒,森本茂文,北田徳昭,瓜生原健嗣,橋田亨:周術期における薬物療法への薬剤師の早期介入とその評価~入院前検査センターにおける投薬マネジメント~.第4回日本薬剤師レジデントフォーラム,東京,2015.02.07
- 藤田和美,池村舞,橋田亨:フェニトインによる肝障害発症時の肝逸脱酵素の評価.4階日本薬剤師レジデントフォーラム,東京,2015.02.07
- 森本茂文:私の考える医療における薬剤師の職能.関西POS薬剤研究会,大阪,2015.02.07
- 平畠正樹:内服抗悪性腫瘍剤における薬剤師の関わりについて.第16回西市民病院薬薬連携オープンカンファレンス,神戸,2015.02.10
- 杉山有吏子:入院前からの薬剤師の取り組み~シームレスな入院を目指して~.第15回神戸糖尿病チーム医療研究会,神戸,2015.02.13
- 杉原千尋,村中幸子,阿河祐二,小曳恵里子,竹中麻理子,東別府直紀,西岡弘晶:脳卒中センターにおける病棟NST活動の報告.第30回日本静脈経腸栄養学会学術集会,神戸,2015.02.13
- 橋田亨: 安心とやりがいの職場づくり~抗がん剤暴露ゼロ作戦とキャリアへの配慮~.大阪市立大・がんプロフェッショナル養成プラン講演会, 大阪, 2015.2.13
- 橋田亨:今、薬剤師に求められている病診薬連携のシームレスな実施について.大阪薬科大学第7回がんプロシンポジウム, 高槻, 2015.2.15
- 中浴伸二:薬物の体内動態、相互作用、薬物血中濃度測定.日本看護協会 平成26年度感染管理認定看護師対象研修プログラム,神戸,2015.02.19
- 森本茂文:がん薬物療法の個別化に向けた取り組み更なる充実-将来を見据える-.国立病院機構平成26年度チーム医療推進のための研修2(がん化学療法),大阪,2015.2.19
- 橋田亨:広がり、深まる薬剤師へのニーズ~医療の大海に向けて宜候~.平成26年度愛知県厚生連薬剤師会総会, 名古屋, 2015.2.21
- 橋田亨:医師と薬剤師の合意に基づく処方提案とアウトカム, 平成26年度厚生労働科学研究費補助金 .薬剤師が担うチーム医療と地域医療の調査とアウトカムの評価研究シンポジウム, 東京, 2015.2.22
- 中浴伸二:薬薬連携を踏まえた持参薬管理 -現状と展望について考える-.兵庫県病院薬剤師会 平成26年度第2回中小病診対策部研修会,神戸,2015.03.05
- 森本茂文:がん医療における「個別化」に薬剤師はどう対応するか?消化器がんにおける「個別化」対応.日本臨床腫瘍薬学会学術大会2015シンポジウム5,京都,2015.03.15
- 野村洋道,平畠正樹,中西真也,八木裕子,辻晃仁,橋田亨:ショートハイドレーション法を用いた高用量シスプラチン投与の安全性.日本臨床腫瘍薬学会学術大会,京都,2015.03.15
- 橋田亨:医薬品の安全な取り扱いを考える.日本医療マネジメント学会第9回兵庫支部学術大会, 小野, 2015.03.15
- 奥貞智:「求められる薬剤師の責任と信頼」~シームレスに薬物治療をつなぐために~.薬剤師のためのWEBシンポジウム,東京,2015.03.16
- 小曳恵里子,柴谷直樹,奥貞智,森本茂文,橋田亨:実践的能力をもつ薬剤師育成のための実習プログラム.日本薬学会第135年会,神戸,2015.03.26
- 安藤基純,小曳恵里子,中浴伸二,柏木裕子,北田徳昭,森本茂文,亀井博紀,園 諭美,西岡弘晶,福田剛史,橋田亨:抗MRSA薬ダプトマイシンの安全性評価と薬物動態への影響因子の解析.日本薬学会第135年会,神戸,2015.03.26
- 平畠正樹:抗癌剤の暴露防止と副作用対策.大阪労災病院オープンカンファレンス,大阪,2015.03.26
- 橋田亨:薬剤師によるがん化学療法の安全管理~抗がん剤職業上暴露と有害反応を制御する~. 日本薬学会第135年会,神戸, 2015.03.26
- 橋田亨:薬剤師外来の構築と地域医療.日本薬学会第135年会, 神戸, 2015.03.28
- 橋田亨:近未来的遠隔医療に求められる薬剤師の役割.日本薬学会第135年会, 神戸, 2015.03.28
2013年度 誌上発表
著書
- 橋田亨,北田徳昭,原田奈生子,長野徹(分担執筆). 11皮膚疾患,77帯状疱疹. 薬局2014年3月増刊号, 薬と検査2014薬物治療&服薬指導プラクティカルガイド. 南山堂, 東京, 990-1003, 2014
論文
- 北田徳昭,金森健悟,小西絢子,田中詳二,杉之下与志樹,猪熊哲朗,橋田 亨: ソラフェニブを用いた肝がん治療の現状と投薬期間に影響を与える因子の解明. 癌と化学療法 40: 479-82,2013
- 大道真由美,北田徳昭,登佳寿子,田中詳二,田路佳範,吉本明弘,鈴木隆夫,福島昭二,橋田亨: 腹膜透析患者における1週間あたりのダルベポエチンアルファ平均投与量とヘモグロビン値との相関性の解析. 日本透析医学会誌 46: 419-26, 2013
- 中浴伸二,北田徳昭,山本健児,有吉孝一,橋田亨: 大規模災害被災地に対する医療支援-救護所における処方動向とグループページを活用した後方支援-.日臨救医誌 16: 589-94, 2013
総説等
- 北田徳昭,橋田亨: これから始める病棟薬剤業務~先行事例からヒントを得る~持参薬をどう管理するか. 大規模病院:シームレスな薬物治療を目指した入院前からの常用薬確認. 月刊薬事 55: 953-7,2013
- 北田徳昭: 症状と対処の仕方がわかる!第9回~抗がん薬の副作用とマネジメント. 乳がん. weekly PTX単独療法. 月刊薬事 55: 1330-6, 2013
2013年度 学会発表・講演
- 小曳恵里子,安藤基純,北田徳昭,中浴伸二,柏木裕子,山本健児,西岡弘晶,橋田 亨: ダプトマイシンの血中濃度モニタリングと安全性に関する検討. 第30回日本TDM学会学術大会,熊本,2013年5月26日
- 土肥麻貴子,池村 舞,中浴伸二,北田徳昭,橋田 亨: 重症部門領域におけるClostridium difficile感染症発生患者の酸分泌抑制薬の使用実態. 医療薬学フォーラム2013/第21回クリニカルファーマシーシンポジウム,金沢, 2013年7月18日
- 柴谷直樹,原田奈生子,永井美帆,北田徳昭,橋田 亨: タブレット端末を用いた医薬品情報室からの病棟薬剤業務支援とその評価. 第16回日本医薬品情報学会総会・学術大会,名古屋,2013年8月10日
- 山本晴菜,北田徳昭,柴谷直樹,平畠正樹,橋田 亨: 新規医薬品導入時におけるリスク最小化のための情報提供のあり方-デノスマブを例に-. 第16回日本医薬品情報学会総会・学術大会,名古屋, 2013年8月11日
- 野村洋道,平畠正樹,中西真也,北田徳昭,辻 晃仁,橋田 亨: 高用量シスプラチンによる腎障害の要因解析. 第11回日本臨床腫瘍学会学術集会,仙台, 2013年8月31日
- 水谷仁美,鶴谷 茂,西岡和子,北田徳昭,山川 勝,橋田 亨: 低出生体重児における高カロリー輸液の特徴とその調製. 第16回日本注射薬臨床情報学会,西宮, 2013年8月31日
- 稲角利彦,金剛圭祐,薩摩由香里,大音三枝子,北田徳昭,梅田節子,李 美於,橋田 亨:緩和ケア外来における薬剤師の介入とその評価. 第7回日本緩和医療薬学会年会,千葉, 2013年9月15日
- 風間 正,北田徳昭,長谷川 豊,橋田 亨,濱口常男: 非ホジキンリンパ腫に対するR-CHOP療法における血液毒性に及ぼす性差の影響. 第23回日本医療薬学会年会,仙台, 2013年9月21日
- 奥貞佳奈子,山本千代,登 佳寿子,北田徳昭,石川隆之,橋田 亨: Bcr-Ablチロシンキナーゼ阻害薬服用患者を対象とした薬剤師外来の必要性. 第23回日本医療薬学会年会,仙台, 2013年9月21日
- 中西真也,平畠正樹,野村洋道,北田徳昭,濱田麻美子,佐藤杏子,佐竹悠良,古武 剛,辻 晃仁,橋田 亨: ホスアプレピタントによる注射部位障害の実態調査と血管痛に対する温罨法の効果. 第51回日本癌治療学会学術集会,京都, 2013年10月25日
- 熊谷美香,池村 舞,中浴伸二,柏木裕子,北田徳昭,橋田 亨: 重症感染症患者におけるバンコマイシン散の腎機能に及ぼす影響. 第35回日本病院薬剤師会近畿学術大会,京都, 2014年2月1日
- 金城奈美,奥貞 智,池村 舞,北田徳昭,瓜生原健嗣,橋田 亨: 周術期における薬物療法への薬剤師の介入基準の明確化とその評価~入院前検査センターにおける抗血栓薬のマネジメント~. 第35回日本病院薬剤師会近畿学術大会,京都, 2014年2月1日
- 米澤武志,奥貞 智,金城奈美,柴谷直樹,北田徳昭,瓜生原健嗣,橋田 亨: 入院前検査センターにおける抗血栓症薬の中止指示の遵守状況. 第35回日本病院薬剤師会近畿学術大会,京都, 2014年2月1日
- 川口奈奈子,原田奈生子,柴谷直樹,北田徳昭,猪熊哲朗,橋田 亨: C型慢性肝炎3剤併用療法における腎機能障害及び貧血の発現状況. 第35回日本病院薬剤師会近畿学術大会,京都, 2014年2月1日
- 橋口文乃,濱 宏仁,樋口弘実,北田徳昭,政井栄久,橋田 亨: 電子カルテアレルギー歴情報抽出システムの構築と病棟薬剤業務への適用. 第35回日本病院薬剤師会近畿学術大会,京都,2014年2月1日
- 中浴伸二,安藤基純,林 卓郎,井上 彰,有吉孝一,北田徳昭,橋田 亨: 維持血液透析中の高齢者がアテノロールの血中濃度上昇により徐脈を来した1例. 第34回日本中毒学会西日本地方会学術集会,枚方, 2014年2月22日
- 山本千代,奥貞佳奈子,登 佳寿子,北田徳昭,入江 慶,岡田 裕,平本展大,橋本尚子,石川隆之,橋田 亨: 同種造血幹細胞移植前処置におけるニューロキニン1受容体拮抗薬投与の免疫抑制剤の血中濃度に及ぼす影響. 第36回日本造血細胞移植学会総会,沖縄,2014年3月8日
- 柴谷直樹,仲宗根亜紀,佐藤志保,岸本修一,北田徳昭,西岡和子,青木卓哉,北 正人,猪爪信夫,橋田 亨,福島昭二: 切迫早産患者におけるリトドリンの全身クリアランスに及ぼす遺伝子多型の影響. 日本薬学会第134年会,熊本, 2014年3月30日
- 北田徳昭: 抗がん薬の投与マネジメント. 神戸市立医療センター中央市民病院がん診療オープンセミナー,神戸, 2013年9月12日
- 橋口文乃,濱 宏仁,樋口弘実,北田徳昭,政井栄久,橋田 亨: 電子カルテアレルギー歴情報抽出システムの構築と病棟薬剤業務への適用. 第3回薬剤師レジデント交流会,西宮, 2014年3月21日
- 川口奈奈子,小曳恵里子,原田奈生子,柴谷直樹,北田徳昭,橋田 亨: C型慢性肝炎3剤併用療法における貧血および急性腎不全の発現状況. 第3回薬剤師レジデント交流会,西宮, 2014年3月21日
- 熊谷美香,池村 舞,山本晴奈,中浴伸二,柏木裕子,北田徳昭,橋田 亨: 重症感染症患者におけるバンコマイシン散経口投与による腎機能への影響. 第3回薬剤師レジデント交流会,西宮,2014年3月21日
- 清水里沙,山本千代,奥貞佳奈子,北田徳昭,橋田 亨: 神戸市立医療センター中央市民病院での薬剤師レジデント研修プログラムを終えて. 第3回薬剤師レジデント交流会,西宮,2014年3月21日
- 笠井祐希,酒井麻衣,池村 舞,平畠正樹,北田徳昭,橋田 亨: ボルテゾミブ皮下投与による末梢神経障害の発現状況と薬剤師の視点から見えてきた問題点. 第3回薬剤師レジデント交流会,西宮,2014年3月21日
- 平野達也,高瀬友貴,藤原秀敏,北田徳昭,橋田 亨: 間質性肺炎におけるネオーラル®の服用2時間後血中濃度測定と腎障害に関する検討. 第3回薬剤師レジデント交流会,西宮,2014年3月21日
- 高橋亜里紗,永井美帆,平畠正樹,北田徳昭,橋田 亨: 経口慢性骨髄性白血病治療薬における化学療法により発症するB型肝炎対策の現状. 第3回薬剤師レジデント交流会,西宮,2014年3月21日
- 金城奈美,米澤武志,奥貞 智,池村 舞,北田徳昭,橋田 亨: 入院前検査センターにおける抗血栓薬内服患者への薬剤師の介入基準の導入とその評価. 第3回薬剤師レジデント交流会,西宮,2014年3月21日
- 北田徳昭:薬学的介入の実際と症例サマリ-消化器がん-. 日本医療薬学会第1回がん専門薬剤師認定申請のための症例サマリ書き方講座,東京,2013年5月19日
- 橋田亨:病棟薬剤業務の新展開を担う組織づくりと薬剤師の養成、Pharmaceutical Management Meeting 2013、東京、2013年5月25日
- 橋田亨:新世代薬剤師が担う病院業務、第36回徳島大学薬学部卒後教育公開講座、徳島、2013年6月1日
- 橋田亨:病院薬剤師の新しい業務展開とキャリアパス、山口県病院薬剤師会薬学研究会、山口、 2013年6月2日
- 中浴伸二:「ダプトマイシン投与患者における血中濃度モニタリングの試み」、MRSA感染症セミナー、神戸、2013年7月10日
- 北田徳昭:外来診療におけるチーム医療-経口抗がん薬の投与マネジメント. 第6回淡路島がん診療連携推進セミナー,洲本,2013年7月25日
- 橋田亨:抗がん薬のトータルマネジメント、近畿ブロックがん化学療法認定薬剤師講習会、大阪、 2013年8月24日
- 橋田亨:チーム医療を推進する新時代の薬剤師、岩手医科大学薬学部第1回卒後研修講座、盛岡、2013年8月25日
- 橋田亨:がんのチーム医療と薬剤師の役割—がん専門薬剤師について、大阪薬科大学第3回がんプロシンポジウム、高槻、2013年8月26日
- 橋田亨:注射抗がん薬のトータルマネジメント、第16回 日本注射薬臨床情報学会、西宮、 2013年8月31日
- 北田徳昭:病薬連携を利用した経口抗がん薬の投与マネジメント. 第1回ポートアイランド薬薬連携の会,神戸,2013年9月11日
- 中浴伸二:「感染制御システムについて、薬剤師の立場から」日本医療情報学会関西支部2013年度秋期講演会・関西医療情報処理懇談会第47回例会、大阪、2013年9月14日
- 橋田亨:病棟薬剤業務の新展開を担う組織づくりと薬剤師の養成、奈良県病院薬剤師会第93回学術講演会、奈良, 2013年9月26日
- 橋田亨:薬剤業務マネジメントと人材養成 〜病院運営の視点を持って〜, 関信地区国立病院薬剤師会・第5回薬剤部科管理者研修会、東京、 2013年10月5日
- 奥貞智:「糖尿病の薬物療法について」糖尿病療養指導士兵庫県連合会主催糖尿病教育セミナー2013、神戸、2013年10月14日
- 北田徳昭:周術期のリスク回避を目指した薬学的マネジメント. 第19回バイエル医療薬学セミナー神戸,神戸,2013年10月16日
- 橋田亨:病院と地域のシームレスな薬物治療をめざして,薬剤分科会 特別シンポジウム, 第52回全国自治体病院学会 in KYOTO、京都、 2013年10月18日
- 北田徳昭:がん薬物療法のトータルマネジメント. 第36回岡山県病院薬剤師会癌薬物療法研究会,岡山,2013年10月19日
- 橋田亨:病院薬剤業務の新展開〜広がるニーズと人材養成〜、第38回日赤臨床薬学研修会、名古屋、2013年11月3日
- 橋田亨:抗がん薬のトータルマネジメント、広島県病院薬剤師会学術講演会、広島、2013年11月15日
- 平畠正樹:「最適レジメンの提案」、Meet The Specialist(日本イーライリリー/兵庫県病院薬剤師会)神戸、2013年11月27日
- 橋田亨:病棟薬剤業務の新展開を担う組織作りと薬剤師の養成、済生会平成25年度薬剤部長研修会、東京、2013年12月13日
- 稲角利彦:シームレスながん患者サポート~薬剤師に求められること~「がん患者の疼痛マネジメント」、第4回兵庫県薬剤師会研修部主催講演会、神戸、2013年12月15日
- 中浴伸二、小曳恵理子:「抗MRSA薬の選択とTDM」、西神戸医療センター臨床検査技術部オープンカンファレンス、神戸、2014年3月5日
- 北田徳昭:がん薬物療法のトータルマネジメント.第3回滋賀県がん薬物療法conference,守山,2014年3月5日
『薬剤師レジデントマニュアル第3版』医学書院,東京,2021, 橋田 亨, 西岡弘晶(編集).

『薬剤師レジデントの鉄則』橋田 亨, 西岡弘晶(編集).医学書院,東京,2016

採用情報
業務内容

教育体制

はたらく環境について
・セントラル、病棟部門等の各部門が自主的に、そして部門横断的に実践できるように整備しています。
・ロボット、IoT技術を活用し、業務の効率化をすすめています。
・薬剤部幹部会議、全員会議、教育チーム会議を定期的に開き、情報を共有し、意見を出し合うことで、薬剤師業務ならびに教育効果を検証し、さらなる改善につなげています。

先輩からのメッセージ