Our department
The Department of Molecular Imaging Research at Kobe City Medical Center General Hospital is pioneering PET clinical trials and related research with the highest level of technology and experience.
The term “molecular imaging” refers to the visualization of activity at the cellular or molecular level in vivo. Our department is working to elucidate the pathophysiology of disease and develop new drugs using a diagnostic modality called “PET,” that allows the visualization of biological function.
Our PET responsibilities include manufacturing investigational drugs, conducting clinical research, developing imaging and analysis methods, and maintaining quality control.
As a department of public municipal hospital, we are also working to provide our patients with cutting-edge achievement obtained through clinical studies. We will contribute the advancement of medical care by developing safe and high effective diagnostic techniques using PET.
PET drug manufacturing
Japan’s leading GMP system for PET investigational drugs
Our department started conducting PET clinical trials in 2008, and established a new GMP-compliant hot lab for the development of investigational new drugs in 2011*1. These achievements and infrastructures have been highly evaluated, and domestic and foreign pharmaceutical companies have contracted us to manufacture various PET investigational drugs. These manufactured drugs are then used within our facility or transported to nearby medical institutions for PET clinical trials.
Our department has a self-shielded cyclotron and two GMP-compliant hot lab areas for PET drug production (one for new investigational drugs and one for research). The production of PET drugs for clinical trials, including PET diagnostic drug trials and therapeutic drug trials using PET as a biomarker, requires quality control, quality assurance, and good hygiene practices in accordance with global standards. Since few facilities in Japan can meet these requirements, we are proud to provide a high-quality PET site for such clinical trials.
We are also involved in the production of numerous PET drugs for research purposes. In addition, we are developing new methods for the synthesis of PET drugs.
The manufacturing of PET investigational new drugs is handled by experts in PET drug manufacturing and management who work with a partner company. Based on our experience, we can provide consulting services to client companies regarding drug manufacturing, compliance with Japanese laws and regulations, and the preparation of relevant documents. In addition, we are developing a production facility for nuclear medicine therapeutics labeled with alpha or beta radionuclides to follow the emerging field of ‘theranostics’, which indicates the fusion of diagnosis and treatment that has recently attracted worldwide attention.
*1 This was implemented in the Institute of Biomedical Research and Innovation, a former institute to which our department belonged. It was then transferred and integrated into Kobe City Medical Center General Hospital in November 2017.
- Iimori H, Hashizume Y, Sasaki M, et al. First automatic radiosynthesis of 11C labeled Telmisartan using a multipurpose synthesizer for clinical research use. Ann Nucl Med. 2011;25:333-337.
PET clinical trials
Our experienced staff handle all types of PET clinical trials
PET clinical trials use radioactive drugs whose radioactivity decays over time. Unlike other general pharmaceutical trials, PET trials have special procedures for subject handling, image acquisition, and image processing. In our department, PET clinical trials are fully managed by our experienced staff of physicians, radiological technologists, nurses, and clinical research coordinators.
Our hospital has three PET/CT scanners. One of these is used exclusively for clinical trials and research, allowing us to be flexible with clinical trial schedules. Additionally, we are able to conduct PET clinical trials using 11C and 68Ga in addition to 18F.
- Ohnishi A, Akamatsu G, Ikari Y, et al. Dosimetry and efficacy of a tau PET tracer [18F]MK-6240 in Japanese healthy elderly and patients with Alzheimer’s disease. 2023;37:108-120.
- Nakano M, Nakamura T, Takita Y, et al. Radiation dosimetry and pharmacokinetics of florbetapir (18F) in Japanese subjects. Ann Nucl Med. 2019;33:639-645.
- Miki T, Shimada H, Kim JS, et al. Brain uptake and safety of Flutemetamol F 18 injection in Japanese subjects with probable Alzheimer’s disease, subjects with amnestic mild cognitive impairment and healthy volunteers. Ann Nucl Med. 2017;31:260-272.
- Senda M, Yamamoto Y, Sasaki M, et al. An exploratory efficacy study of the amyloid imaging agent [18F]flutemetamol in Japanese Subjects. Ann Nucl Med. 2015;29:391-399.
- Senda M, Brooks DJ, Farrar G, et al. The clinical safety, biodistribution and internal radiation dosimetry of flutemetamol (18F) injection in healthy Japanese adult volunteers. Ann Nucl Med.2015;29:627-635.
- Senda M, Sasaki M, Yamane T, et al. Ethnic comparison of pharmacokinetics of 18F-florbetaben, a PET tracer for beta-amyloid imaging, in healthy Caucasian and Japanese subjects. Eur J Nucl Med Mol Imaging. 2015;42:89-96.
PET clinical research
Accepting PET research from other institutes
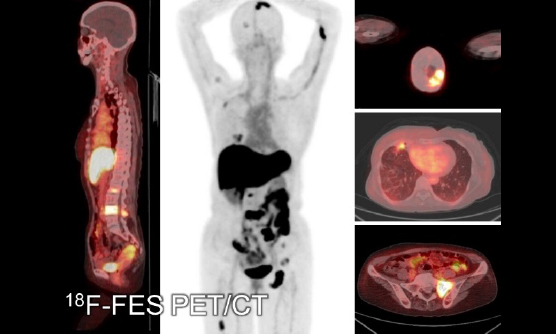
In addition to these clinical trials, we are actively engaged in clinical research projects. The targets of our PET range from brain disorders to malignant tumors. Aside from collaborating with each clinical department of Kobe City Medical Center General Hospital, we also accept patients from other hospitals such as Kobe University Hospital, RIKEN, and the other institute. We also participate in the nationwide multicenter research projects, including J-ADNI and AMED preclinical projects. In addition, we have conducted several first-in-human studies of novel PET radiopharmaceuticals.
We also accept requests for PET imaging and collaborations for research purposes. If you would like to conduct clinical research using PET, but your institution does not have the facilities or support for PET clinical research, please contact us.
- Ohnishi A, Yamane T, Shimizu K, et al. False‑positive [18F]FAPI‑74 uptake caused by blood retention due to external jugular vein thrombus: Pitfall in the early‑phase scan. Eur J Nucl Med Mol Imaging. 2024.
- Senda M, Ishii K, Ito K, et al. A Japanese multicenter study on PET and other biomarkers for subjects with potential preclinical and prodromal Alzheimer’s disease. J Prev Alzheimers Dis. 2021;8;495-502.
- Watanabe Y, Mawatari A, Aita K, et al. PET imaging of 11C-labeled thiamine tetrahydrofurfuryl disulfide, vitamin B1 derivative: First-in-human study. Biochem Biophys Res Commun. 2021;555:7-12.
- Akamatsu G, Ohnishi A, Aita K, et al. A revisit to quantitative PET with 18F-FDOPA of high specific activity using a high-resolution condition in view of application to regenerative therapy. Ann Nucl Med. 2017;31:163-171.
- Ohnishi A, Senda M, Yamane T, et al. Exploratory human PET study of the effectiveness of 11C-ketoprofen methyl ester, a potential biomarker of neuroinflammatory processes in Alzheimer’s disease. Nucl Med Biol. 2016;43:438-444.
- Ohnishi A, Senda M, Yamane T, et al. Human whole-body biodistribution and dosimetry of a new PET tracer, [11C]ketoprofen methyl ester, for imagings of neuroinflammation. Nucl Med Biol. 2014;41:594-599.
- Yamane T, Takaoka A, Kita M, Imai Y, Senda M. 18F-FLT PET performs better than 18F-FDG PET in differentiating malignant uterine corpus tumors from benign leiomyoma. Ann Nucl Med. 2012;26:478-484.
- Maeda K, Ohnishi A, Sasaki M, et al. Quantitative investigation of hepatobiliary transport of [11C]telmisartan in humans by PET imaging. Drug Metab Pharmacokinet. 2019;34:293-299.
- Shimizu K, Takashima T, Yamane T, et al. Whole-body distribution and radiation dosimetry of [11C]telmisartan as a biomarker for hepatic organic anion transporting polypeptide (OATP) 1B3. Nucl Med Biol. 2012;39:847-853.
- Kikuchi M, Yamane T, Shinohara S, et al. 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma. Ann Nucl Med. 2011;25:625-633.
- Yamane T, Kikuchi M, Shinohara S, Senda M. Reduction of [18F]fluoromisonidazole uptake after neoadjuvant chemotherapy for head and neck squamous cell carcinoma. Mol Imaging Biol. 2011;13:227-231.
- Yamane T, Sakamoto S, Senda M. Clinical impact of 11C-methionine PET on expected management of patients with brain neoplasm. Eur J Nucl Med Mol Imaging. 2010;37:685-690.
- Senda M, Kubo N, Adachi K, et al. Cerebral histamine H1 receptor binding potential measured with PET under a test dose of olopatadine, an antihistamine, is reduced after repeated administration of olopatadine. J Nucl Med. 2009;50:887-892.
PET Quality Control
High quality PET images and precise quantification through professional quality control
Our other goal is to provide a new approach to PET quality control and standardization. Image quality, quantitative values, and diagnostic performance in PET imaging and clinical studies are affected by various elements, including the imaging device (PET camera), imaging method, and image evaluation and analysis. Therefore, standardization through appropriate quality control is necessary to establish PET as a reliable and universal examination tool. Moreover, additional information or highly accurate data can be obtained by developing new methods of acquisition or image analysis.
PET-cores in Multicenter Clinical Studies
In multicenter PET clinical studies, PET cameras, acquisition methods, and the experience and system of each facility are inevitably different. Therefore, standardization and data quality control are necessary to build a reliable multicenter PET database to promote PET as an imaging biomarker. We have collaborated with the J-ADNI and other national multicenter clinical research projects on Alzheimer’s disease as the “PET-core” in these efforts by standardizing PET imaging and conducting data quality control throughout the country. As a result, the Japanese Society of Nuclear Medicine later adopted the results of the PET-core as the standard protocol for FDG and amyloid PET in the brain and the certification system for PET imaging facilities. Furthermore, these methods have been disseminated beyond the project, helping to improve the quality of PET facilities nationwide.
- Ikari Y, Akamatsu G, Matsumoto K, Yamane T, Senda M, Fukuchi K, AMED Preclinical AD Study Investigators. Improved correlation of 18F-Flortaucipir PET SUVRs and clinical stages in the Alzheimer disease continuum with the MUBADA/PERSI-based analysis. J Nucl Med Technol. 2024.
- Senda M. Standardization of PET imaging and site qualification program by JSNM: collaboration with EANM/EARL. Ann Nucl Med. 2020;34:873-874.
- Akamatsu G, Ikari Y, Ohnishi A, et al. Voxel-based statistical analysis and quantification of amyloid PET in the Japanese Alzheimer’s disease neuroimaging initiative (J-ADNI) multi-center study. EJNMMI Res. 2019;9:91.
- Akamatsu G, Nishio T, Adachi K, Ikari Y, Senda M. Whole-body biodistribution and the influence of body activity on brain kinetic analysis of the 11C-PiB PET scan. Radiol Phys Technol. 2017;10:464-474.
- Yamane T, Ishii K, Sakata M, et al. Inter-rater variability of visual interpretation and comparison with quantitative evaluation of 11C-PiB PET amyloid images of the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) multicenter study. Eur J Nucl Med Mol Imaging. 2017;44:850-857.
- Akamatsu G, Ikari Y, Nishio T, et al. Optimization of image reconstruction conditions with phantoms for brain FDG and amyloid PET imaging. Ann Nucl Med. 2016;30:18-28.
- Ikari Y, Akamatsu G, Nishio T, et al. Phantom criteria for qualification of brain FDG and amyloid PET across different cameras. EJNMMI Phys. 2016;3:23.
- Yamane T, Ikari Y, Nishio T, Ishii K, Ishii K, Kato T, et al. Visual-statistical interpretation of 18F-FDG-PET images for characteristic Alzheimer patterns in a multicenter study: inter-rater concordance and relationship to automated quantitative evaluation. AJNR Am J Neuroradiol. 2014;35:244-9.
- Ikari Y, Nishio T, Makishi Y, et al. Head motion evaluation and correction for PET scans with 18F-FDG in the Japanese Alzheimer’s disease neuroimaging initiative (J-ADNI) multi-center study. Ann Nucl Med. 2012;26:535-544.
Staff
| Education | M.D. Kyoto University Ph.D. Kyoto University |
|---|---|
| Board Certifications | Diagnostic radiology (Japanese Radiological Society) Nuclear medicine (Japanese Society of Nuclear Medicine) |
| Education | M.D. Nara Medical University Ph.D. Nara Medical University |
|---|---|
| Certifications | Nuclear medicine (Japanese Society of Nuclear Medicine) Diagnostic radiology (Japanese Radiological Society) Fellow of Asian nuclear medicine board (Asian Nuclear Medicine Board) |
| Publications | https://researchmap.jp/tomohiko_yamane/published_papers |
| Education | M.D. Kobe University Ph.D. Kobe University |
|---|---|
| Board Certifications | Psychiatry (Japanese Societry of Psychiatry and Neurology) Geriatric psychiatry (Japanese Psychogeriatric Society) Dementia (Japanese Society of Dementia Research) |
| Education | M.D. Nara Medical University |
|---|---|
| Board Certifications |