greeting
Kobe City Medical Center General Hospital operates 24 hours a day with the basic philosophy of "providing safe, patient-centered, high-quality medical care to protect the lives and health of citizens as a key medical facility in Kobe City." The basic policy is to practice emergency medical care and provide highly advanced medical care. On the other hand, at our hospital, we have been working on clinical trials and clinical research for a long time in order to develop safer and more effective medical care. In November 2017, the Center for Clinical Research and Innovation Research and Innovation was established on the occasion of the integration of the adjoining Advanced Medical Center Hospital. Various laws and regulations are established to conduct clinical trials and clinical research. It is strictly required to understand them correctly, to protect subjects, to ensure data reliability, ethics and scientific validity, and to make funding and support transparent. In order to protect the rights and safety of patients and actively contribute to the development of new medical care, we will continue to strive to expand the organization and functions that manage and support clinical trials and clinical research. We appreciate your support and cooperation.
Toru Hashida Director, Clinical Research and Innovation
Philosophy and business
Philosophy
- By actively promoting clinical research, we will contribute to improving the health of citizens by providing cutting-edge medical care as soon as possible, while creating new medical care that will lead to maintenance of life and improvement of quality of life. Contribute to development.
- In order to properly conduct clinical research, including clinical trials of pharmaceuticals and medical devices, etc., we will ensure ethics and scientificity in accordance with laws and guidelines, and manage research so that research can be conducted smoothly and safely. and assist the subject.
business
- Promotion and management of corporate clinical trials for pharmaceuticals and doctor devices, and investigator-initiated clinical trials
- Promotion and management of doctor-initiated clinical research centered on specific clinical research
- Counseling and support for researchers and clients
- Subject protection and support services
- Education related to clinical research promotion
- Operations related to promotion of clinical research at other medical institutions
- Other necessary business
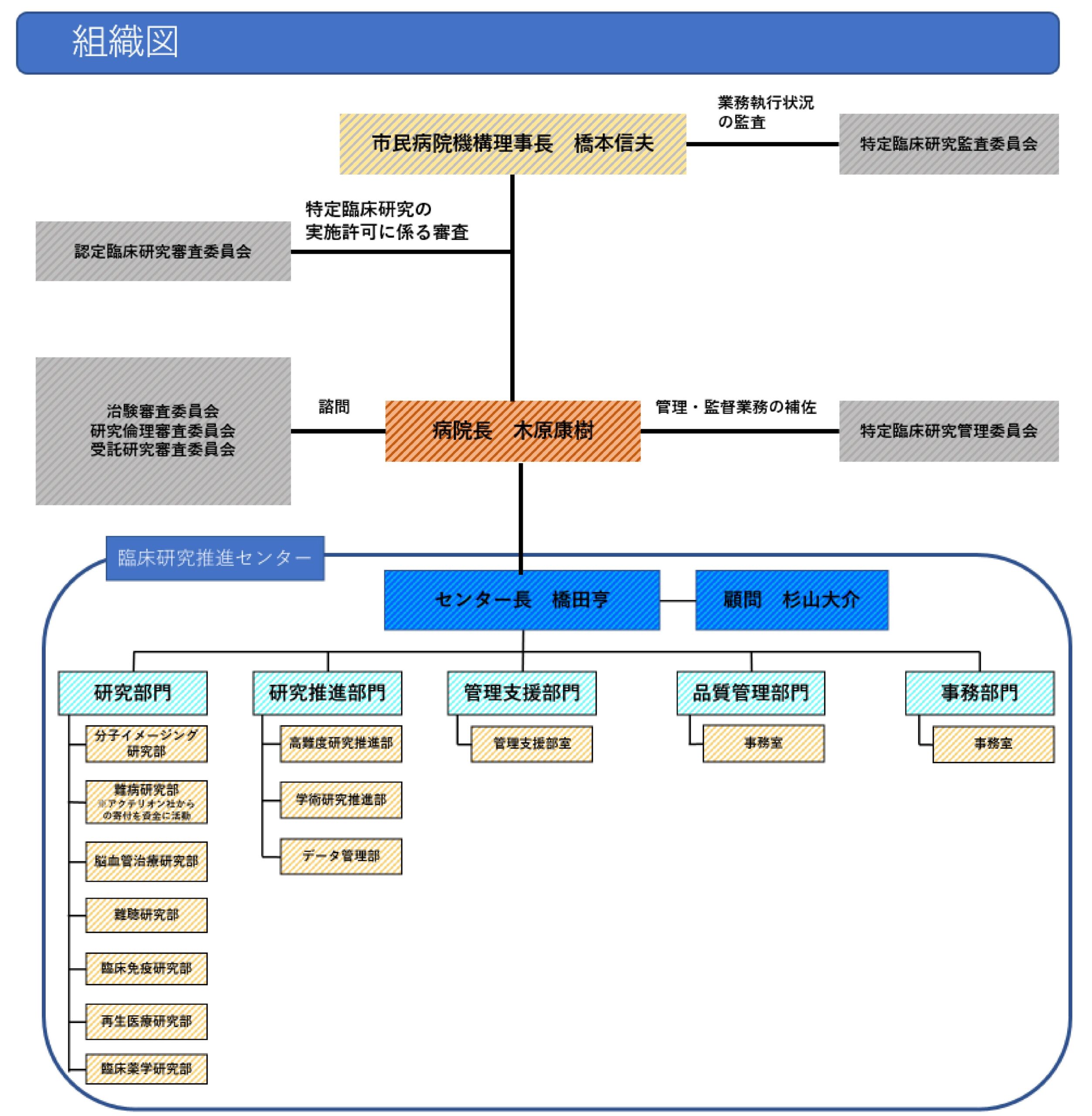
Organization chart
Activity policy and activity details
research department
Molecular Imaging Research Department
Molecular imaging is the visualization of cellular and molecular level activities taking place within the body. The Molecular Imaging Research Department is working to elucidate the pathology of diseases and develop new drugs by utilizing a diagnostic technology called "PET" that visualizes biological functions.
The main missions of our research department are the manufacturing of investigational drugs and the implementation of clinical trials related to PET, clinical research, and the development and quality control of imaging and analysis methods. As a municipal hospital, we also strive to create a system that provides the public with cutting-edge research results obtained through clinical research. We will make maximum use of the know-how we have cultivated in drug discovery, imaging, and quality control to develop safe and highly effective diagnostic techniques using PET, and contribute to the advancement of medicine.
Intractable Disease Research Department
The intractable disease research department uses donations from Actelion Pharmaceuticals Japan Co., Ltd. (currently Janssen Pharma K.K.) to conduct genome analysis and gene expression analysis on patients suspected of having genetic abnormalities for diagnosis. In addition to clarifying the onset mechanism after anonymizing this information, we also worked on intractable diseases such as Parkinson's disease and amyotrophic lateral sclerosis, acute myeloid leukemia, oral cancer, and anemia-complicated heart failure. We are also engaged in research aimed at developing new treatments and diagnostic methods for diseases.
Neurovascular Research
We are actively working with researchers and companies to manage clinical trials and multi-center joint research on cerebrovascular treatment and develop medical devices related to cerebrovascular treatment (device development research).
Hearing Research
Optical topography (fNIRS) that can be performed even in children with cochlear implants, focusing on (1) the development of the visual cortex (response to audio stimuli) and (2) audiovisual integration (synchronous activity between the auditory and visual cortices) after cochlear implant surgery We will conduct clinical research to evaluate brain function using a multi-channel electroencephalograph and lead to the development of new rehabilitation programs.
Rheumatology
To promote the clinical development of "anti-PD-1 agonist antibody therapy" and "early diagnosis marker for inflammatory diseases" being researched by the Immunology Research Department, Advanced Medical Research Center, Kobe Medical Industry Development Organization , in cooperation with the same research department, build related clinical infrastructure, plan, implement and manage clinical research, build evidence related to immune diseases, etc., and provide feedback for the development of new therapeutic methods and diagnostic methods. increase.
Regenerative Medicine
We are conducting research on cell transplantation therapy for central nervous system diseases using stem cells, and are working on research on post-transplantation immune response control. First of all, we will promote the clinical application of regenerative medicine for Parkinson's disease in the field of neurology, which is our specialty. .
Click here for detailsDepartment of Clinical Pharmacy
The Clinical Pharmacy Research Department finds solutions to various themes related to the appropriate use of medicines through clinical pharmacy research, proposes and implements them in actual clinical settings, and verifies their effectiveness.In addition, we provide clinical pharmacological support to doctor in their clinical research by proposing pharmacokinetic and pharmacodynamic (PK/PD) protocols, measuring drug concentrations, and carrying out pharmacokinetic analysis.
Additionally, based on the "Agreement on Educational and Research Cooperation" concluded with the Kobe Gakuin University Graduate School of Pharmaceutical Sciences, we will also work to develop researchers.
Research Promotion Division
Advanced Research Promotion Department
The Advanced Research Promotion Department is a department that provides specialized support for planning and operation when our doctor are in charge of the coordinating secretariat for investigator-initiated clinical trials and the principal investigator/research secretariat for doctor joint research. These clinical trials and research must be planned and implemented based on the Pharmaceuticals and Medical Devices Law, the Clinical Research Act, ethical guidelines, etc., and the necessary system must be constructed and research conducted based on legal guidelines. It is necessary to report to the researcher, the medical institution, and the authorities as necessary. We will support the work necessary for conducting such research.
Academic Research Promotion Department
The Academic Research Promotion Department was established by merging the former Academic Support Center with the Clinical Research and Innovation. The former Academic Support Center supported various in-hospital initiatives related to academic Clinical Research and Innovation. It is now possible.
Data management department
Administrative support department
Management support department
In order to conduct ethical and scientifically high-quality clinical trials (trials) safely and smoothly, the Administration Support Department is centered on a management department staffed by staff with specialized knowledge of clinical research and a clinical research coordinator (CRC). It consists of support departments that have I would like to contribute to the provision of high-quality medical care while protecting the rights and safety of patients by making full use of my advanced expertise.
Quality control department
Quality control department
The Quality Control Department is a department whose purpose is to ensure the quality of clinical research. Collaborate with various departments within the hospital and appropriately manage clinical research conducted by researchers based on various legal guidelines as a hospital to protect patients who participate in clinical research and ensure reliable clinical research. We will strive to carry out
Administrative department
administrative department
The administration department is in charge of general clerical work such as contracts related to clinical research and trials, document management, aggregation of safety information, intellectual property management, income and expenditure management, public relations and planning coordination, and the "KOBE Monowager Network Secretariat". increase. In addition, we supervise clerical work within the Clinical Research and Innovation and work closely with other departments to provide clerical support for the smooth implementation of clinical research and trials.
Click here for the Kobe Monowager Network official websiteActivity status/results
Clinical trial results (by fiscal year)
| year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| New company clinical trial | 19 | 27 | 30 | 47 | 31 | 29 | 75※1 | 35 | 30 | 31 | 42 | 36 |
| New doctor-initiated clinical trial | 1 | 0 | 1 | 1 | 1 | 3 | 7 | 3 | 3 | 6 | 5 | 3 |
| Total number of implementations | 42 | 59 | 81 | 109 | 116 | 123 | 175 | 173 | 167 | 169 | 180 | 180 |
*1: Includes clinical trials inherited due to the integration of the Advanced Medical Center Hospital in November 2017
Main clinical departments and target diseases
| Cardiology | Chronic heart failure, atrial fibrillation, peripheral arterial disease, etc. |
|---|---|
| Diabetes/ Endocrinology | diabetic kidney disease, diabetic neuropathy |
| Nephrology | Renal anemia, acute kidney injury |
| Neurology | Parkinson's disease, POEMS syndrome, Guillain-Barré syndrome, amyotrophic lateral sclerosis, etc. |
| Gastroenterology | Chronic hepatitis B, chronic hepatitis C, duodenal ulcer, reflux esophagitis, Crohn's disease, etc. |
| Respiratory Medicine | Lung cancer, asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, etc. |
| Hematology | Malignant lymphoma, multiple myeloma, leukemia, myelodysplastic syndrome, myelofibrosis, graft-versus-host disease (GVHD), etc. |
| Oncology | 胃癌、大腸癌、食道癌、膵癌、頭頸部癌、原発不明癌、肺癌、抗がん剤による支持療法など |
| Palliative Care | cancer pain |
| Neuropsychiatry | Alzheimer's dementia |
| Pediatrics | childhood bronchial asthma |
| Dermatology | Atopic dermatitis, postherpetic neuralgia |
| Surgery and Transplant Surgery | surgical field disinfection |
| Breast Surgery | breast cancer |
| Neurosurgery | Stroke, cerebral aneurysm, carotid artery stenosis, etc. |
| Orthopedic Surgery | Osteoarthritis, refractory fracture |
| Obstetrics and Gynecology | cervical cancer, uterine fibroids |
| Urology | Prostate cancer, bladder cancer, renal cell carcinoma, etc. |
| Otorhinolaryngology | hearing loss |
| Anesthesiology | Postoperative sedation |
| Diagnostic Radiology | Alzheimer's dementia (diagnosis), PET examination contract test |
| emergency department | Tachyarrhythmia associated with sepsis |
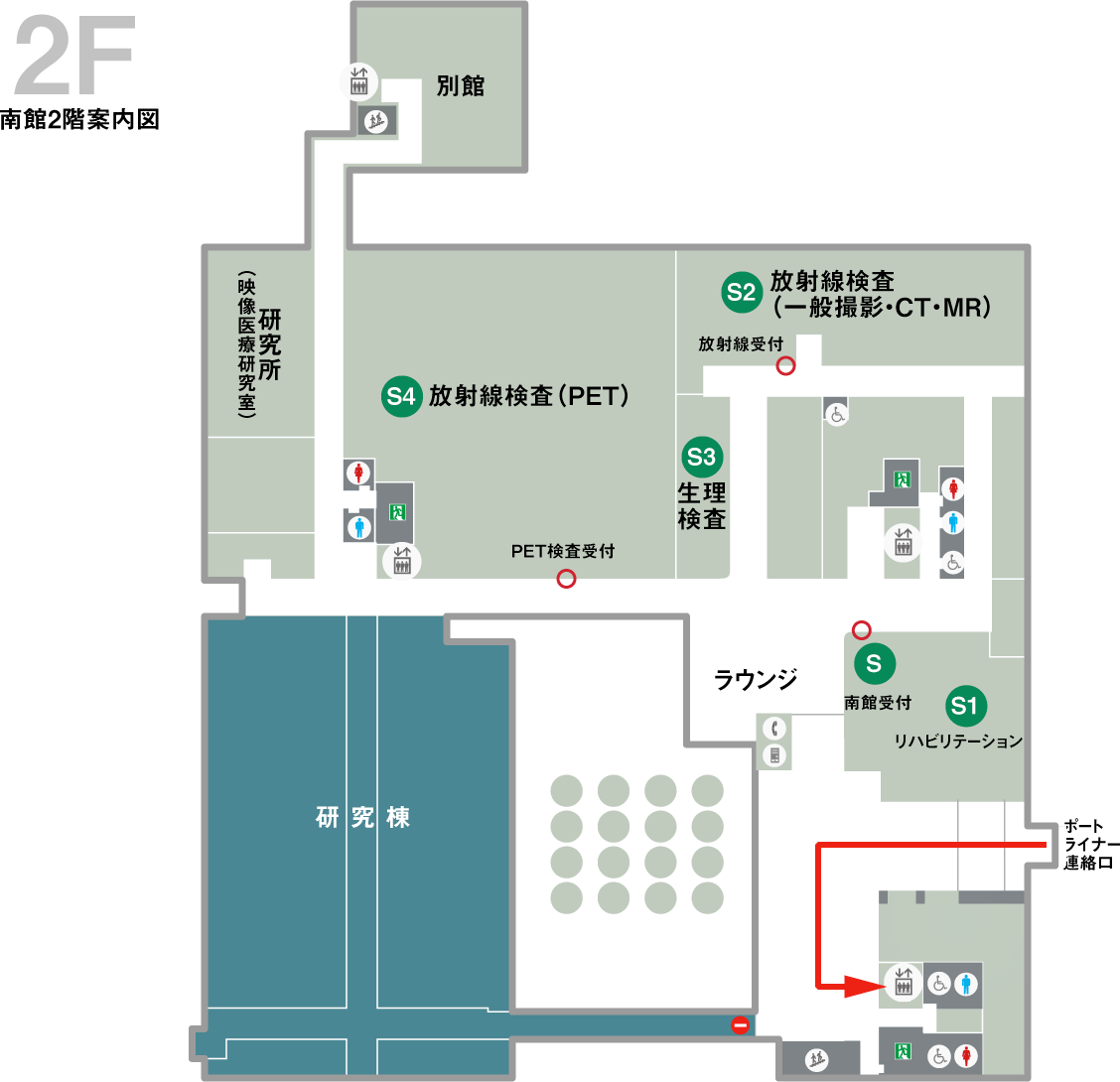
Access to the center
The Clinical Training Promotion Center is located in the main building and the south building of the Kobe City Medical Center.
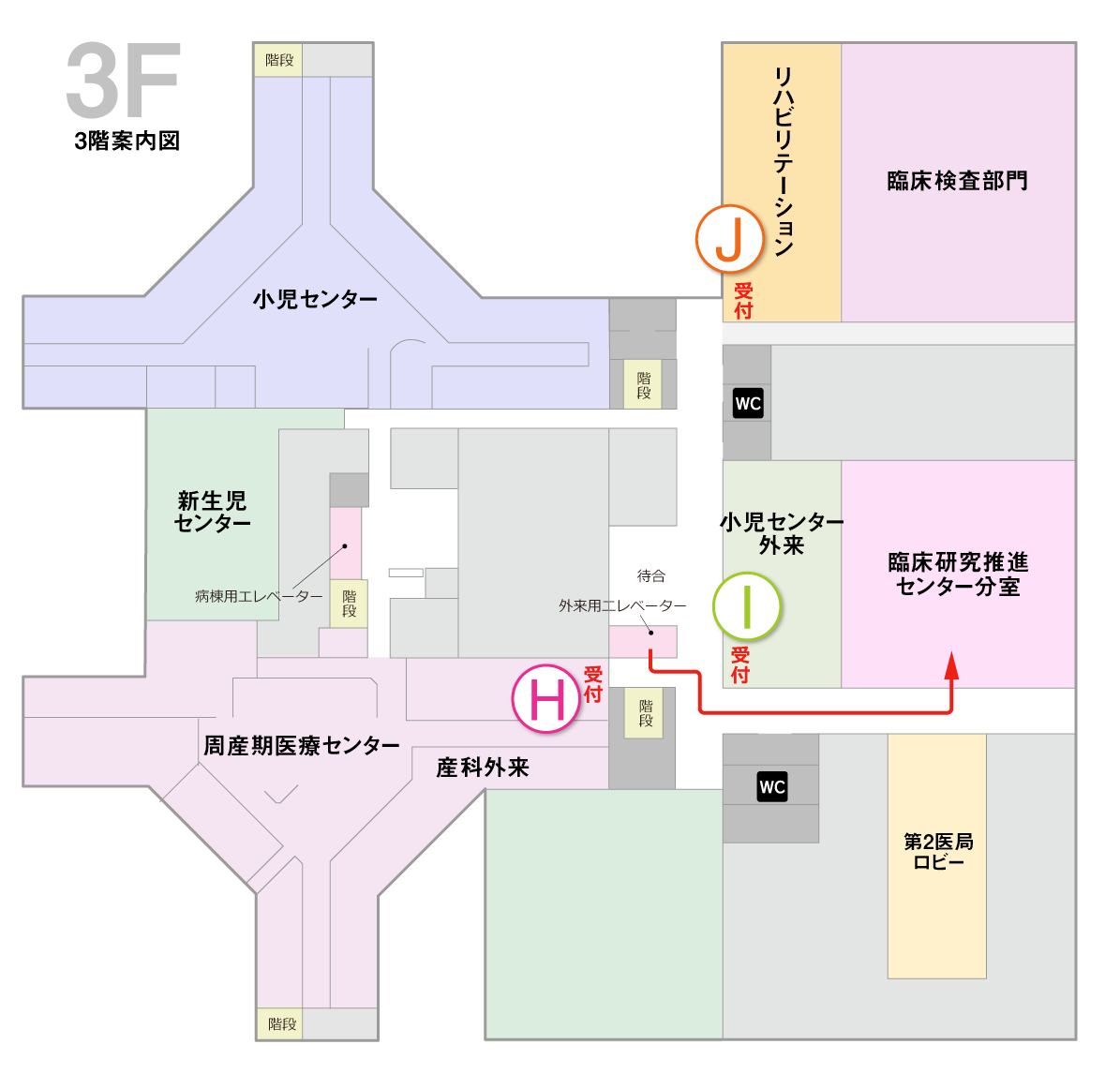
If you are a clinical research coordinator (CRC) at our hospital, please come to the following branch office.
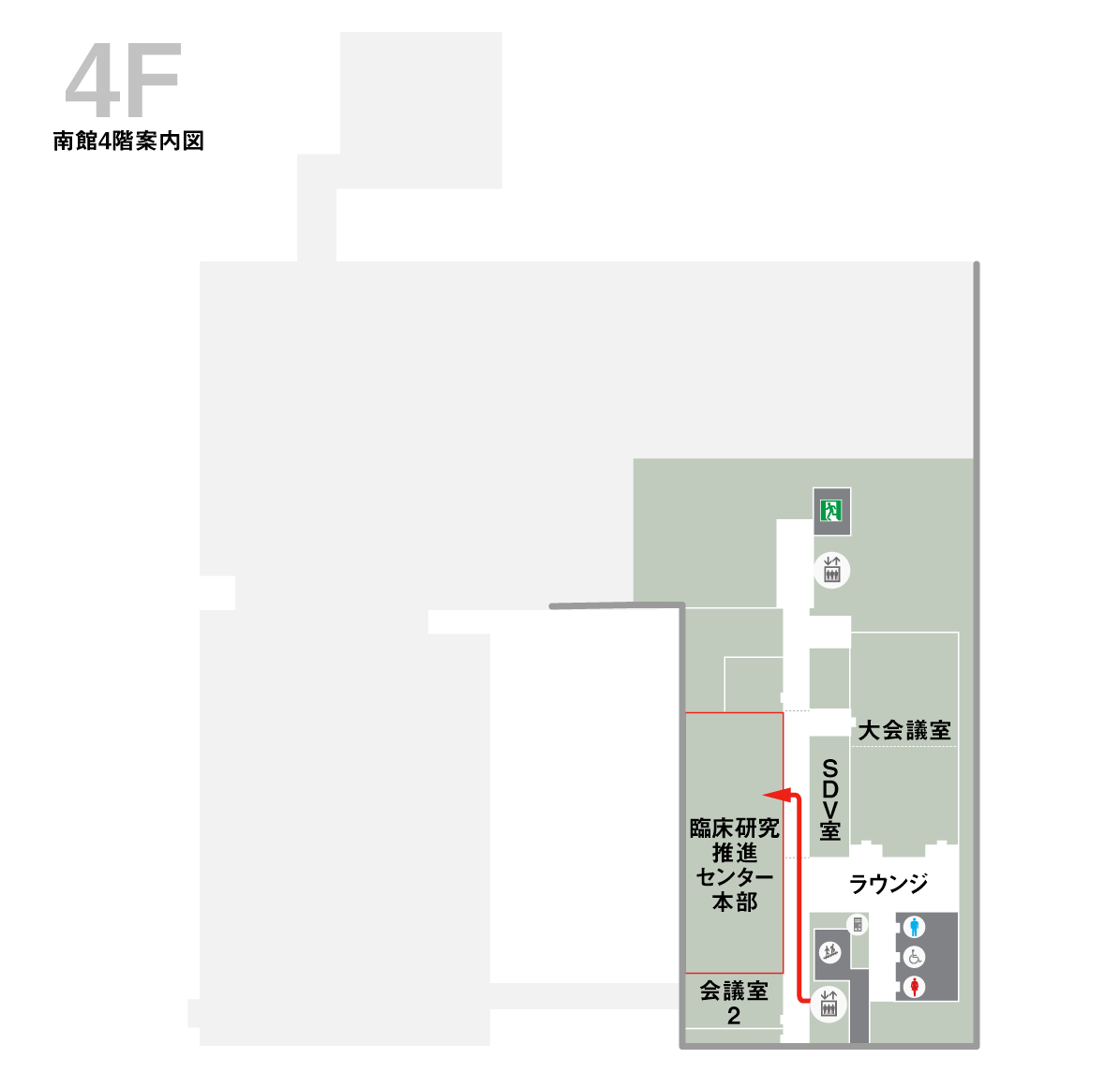
Click here for transportation access to the hospital.Clinical Research and Innovation Headquarters (South Building 4F)
〒650-0047
Minatojima Minamimachi Chuo-ku, Kobe City
Enter the port liner entrance and take the elevator on the left to the 4th floor.
Clinical Research and Innovation Branch Office (Main Building 3F)
〒650-0047
Minatojima Minamimachi Chuo-ku, Kobe City
inquiry
Patient consultation desk for clinical trials and clinical research
〒650-0047
Minatojima Minamimachi Chuo-ku, Kobe City
Clinical Research and Innovation
TEL: (078) 302-4448 *Telephone reception hours are 9:00-17:00 on weekdays
Fax: (078) 302-4604
Email: c_ccri1at-kcho.jp
*To prevent spam, please replace "at" with @ in the email address.
Clinical trial contact information from the sponsor
TEL:078-302-4499 ※電話応対は平日9:00~17:00
Mail:c_ccriあっとkcho.jp
*To prevent spam, please replace "at" with @ in the email address.
Contact information for clinical research from researchers and companies
TEL:078-302-4499 ※電話応対は平日9:00~17:00
Mail:rinkenあっとkcho.jp
*To prevent spam, please replace "at" with @ in the email address.
Contact information for specific clinical research from researchers and companies
TEL:078-302-4499 ※電話応対は平日9:00~17:00
Mail:c_tokuteiあっとkcho.jp
*To prevent spam, please replace "at" with @ in the email address.
Point of contact for general post-marketing surveillance
TEL:078-302-4499 ※電話応対は平日9:00~17:00
Mail:chikenあっとkcho.jp
*To prevent spam, please replace "at" with @ in the email address.