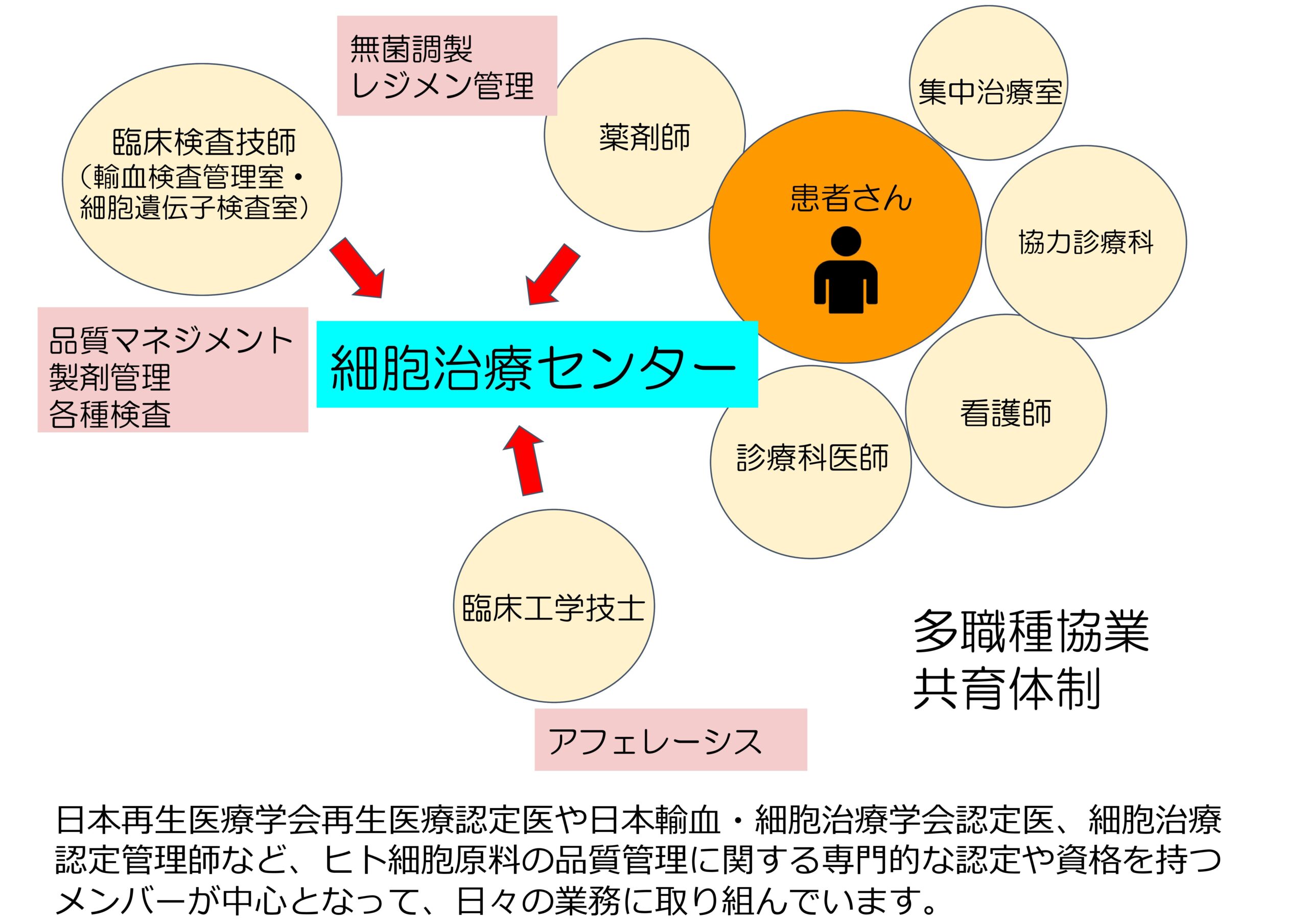
CAR-T療法に代表される細胞治療にはドナー細胞準備のための採取、調製、移植、移植後の管理など、専門的な体制が必要です。細胞治療センターが各診療科と連携し、サポートしていくことで、患者さんに適切な治療を提供していきます。また、近年注目されている幹細胞を用いた再生医療についても治験や臨床研究が行われる際には、細胞の管理や各部署の調整を行い、各診療科をサポートします。異なる細胞治療にはそれぞれに専門的な準備が必要ですが、それらのプラットホームを作り、ノウハウを共有することで、治療の有効性、安全性を高めることができると考えられます。
細胞治療とは
自分もしくは他人由来の細胞を使って、癌を治療したり、失われた機能を再生するために行う治療です。
白血病に対するCAR-T治療、造血幹細胞治療、脊髄損傷に対する骨髄幹細胞治療、神経難病に対する幹細胞治療、などがあります。
CAR-T治療については血液内科のHPをご参照ください。
細胞治療センター
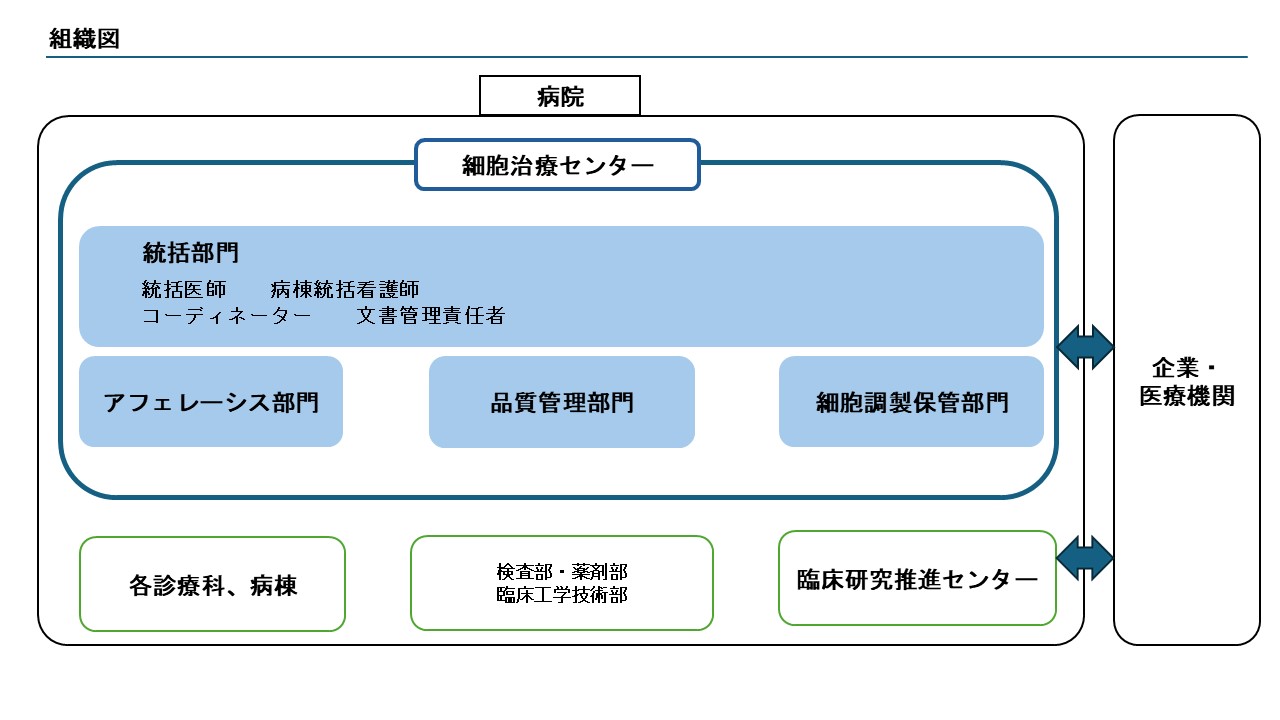
当センターは2024年11月に設立され、診療科医師、臨床検査技術部、薬剤部、臨床工学技術部などの多職種で構成されています。
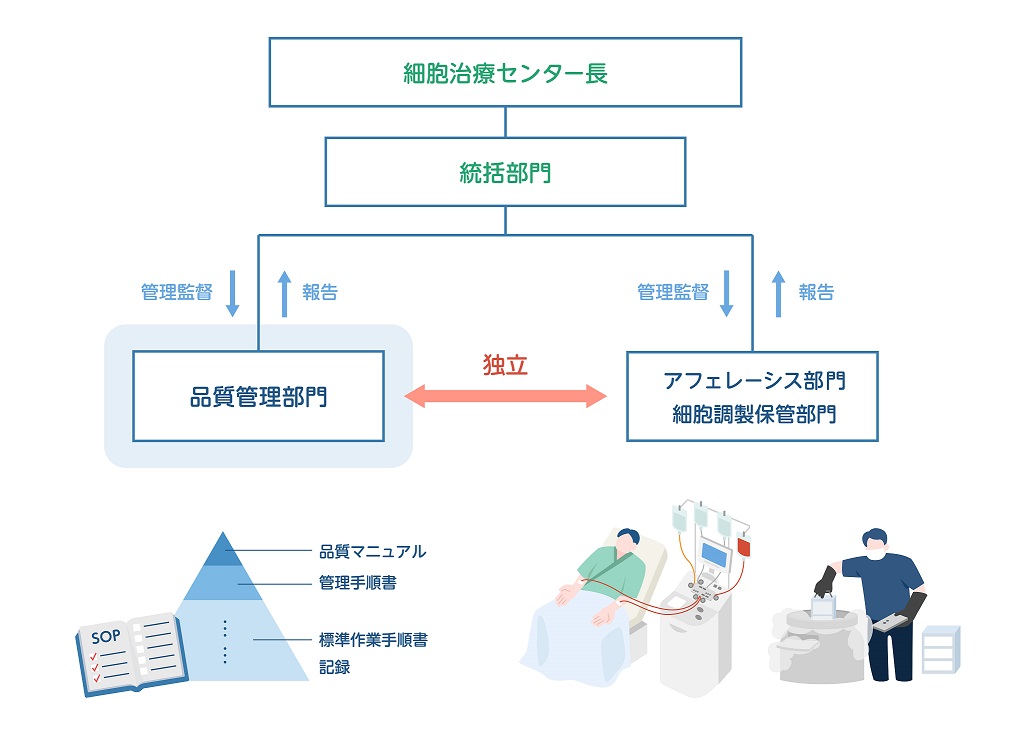
ヒト細胞原料の提供において、品質マネジメントシステムの確立は不可欠であるため、ISO15189を取得した検査部を中心にGMP/GCTP省令に適合した品質マニュアルや管理手順書を作成しています。
- GMP(Good Manufacturing Practice)
医薬品及び医薬部外品の製造管理及び品質管理 の基準に関する省令 - GCTP(Good Gene, Cellular, and Tissue-Based Products Manufacturing Practice)
再生医療等製品の製造管理及び品質管理の基準に関する省令
1.組織・人員体制の整備: 責任者の明確化、専門人材
の配置、スタッフ教育の徹底。
2.文書化と記録管理: 手順書等の文書化、記録の正確
な作成・保管、管理システムの構築。
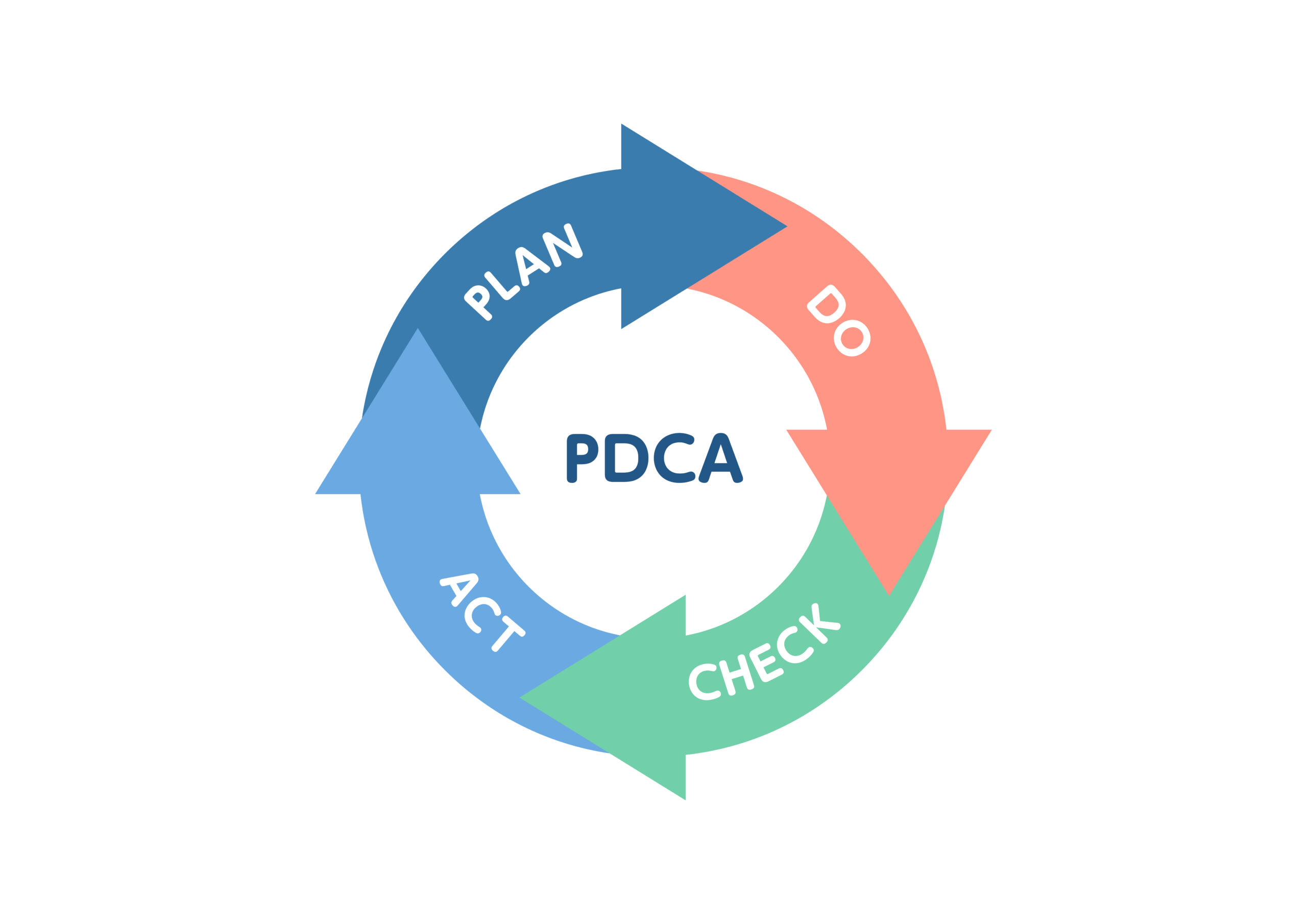
3.PDCAサイクルによる継続的な改善: 課題・改善点の
把握・分析、改善策の実行・検証、標準化による再
発防止。
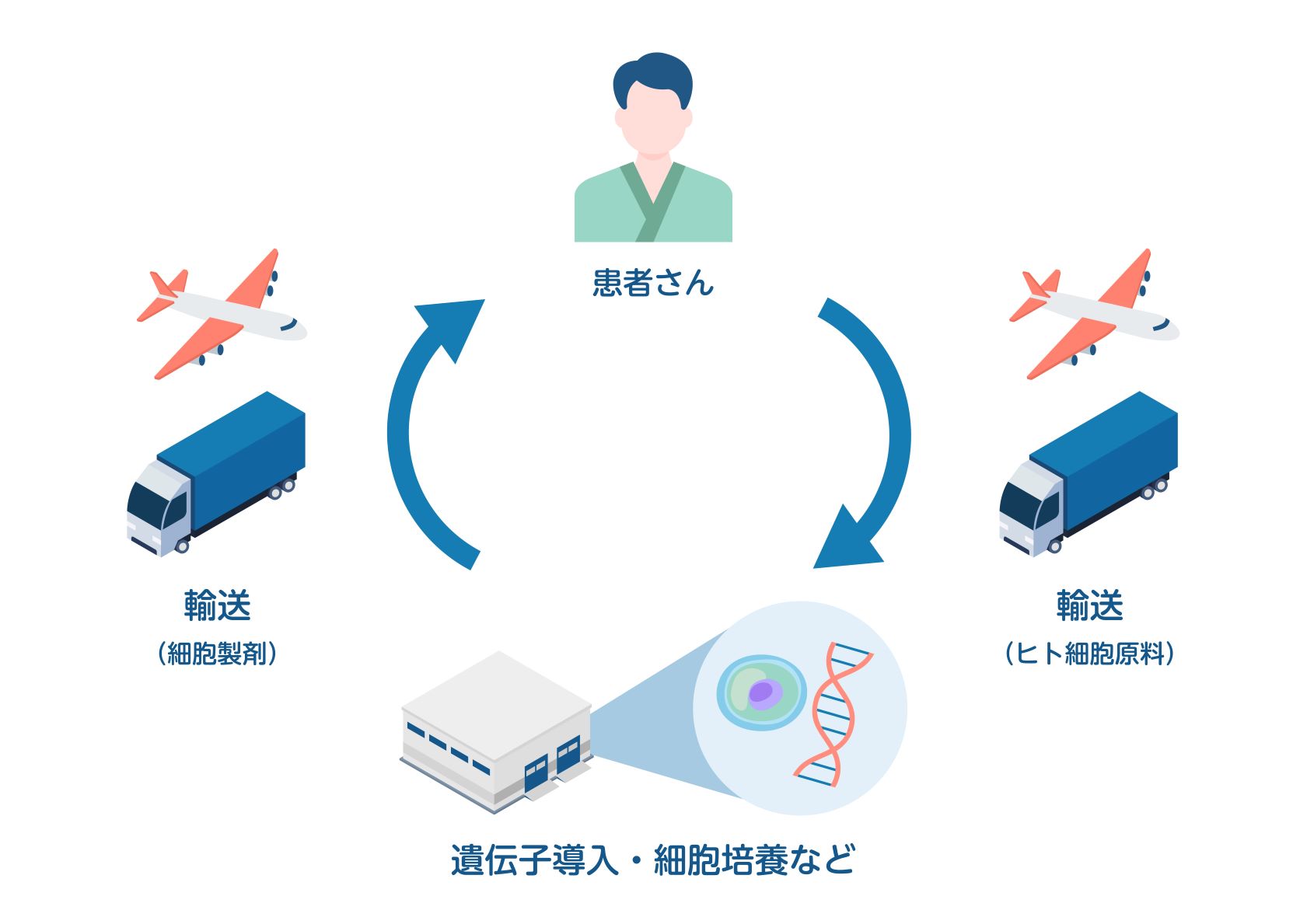
4.トレーサビリティ:患者から採取した細胞が製品化され再び患者本人に戻る(chain of identity:COI)ことをシステムを用いて正しく管理。
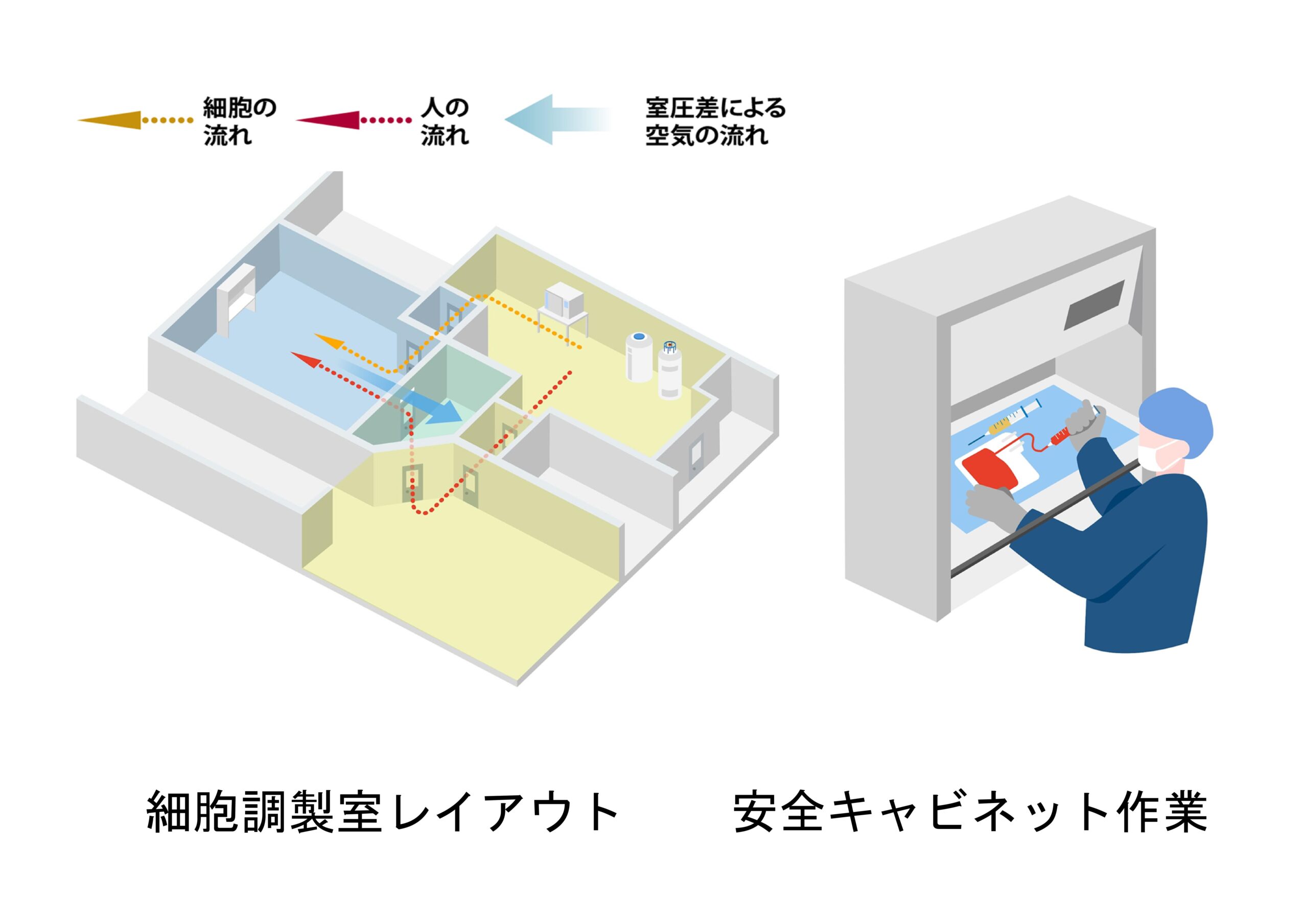
集、関連法規・ガイドラインの遵守。
これらの取り組みを通じて、ヒト細胞原料の品質を確保し、安全で信頼性の高い製品を提供することが可能となります。
組織図
スタッフ
| 氏名 | 所属学会・専門資格等 | |
| センター長 | 森実 飛鳥 | 所属学会 日本脳神経外科学会 日本脳神経外科コングレス 日本脳神経血管内治療学会 日本再生医療学会 国際幹細胞学会 専門医資格 脳神経外科専門医 脳血管内治療専門医 学位 京都大学大学院医学研究科 博士(医学) |
| 統括部門長 |
近藤 忠一 | 所属学会 日本内科学会 日本血液内科学会 日本造血・免疫細胞療法学会 American Society of Hematology 専門医資格 総合内科専門医 血液専門医 学位 京都大学大学院医学研究科 博士(医学) |
| 品質管理部門責任者 | 米谷 昇 | 所属学会 日本内科学会 日本血液学会 日本造血・免疫細胞療法学会 日本輸血・細胞治療学会 専門医資格 総合内科専門医 血液専門医 |
| アフェレーシス部門責任者 | 永井 雄也 | 所属学会 日本内科学会 日本血液学会 American Society of Hematology 専門医資格 総合内科専門医 血液専門医 学位 京都大学大学院医学研究科 博士(医学) |
| 細胞調製保管部門責任者 | 平本 展大 | 所属学会 日本内科学会 日本血液学会 日本造血・免疫細胞療法学会 日本臨床腫瘍学会 専門資格 認定内科医 血液専門医 |
| アフェレーシス部門責任者補佐 | 山本 隆介 | 所属学会 日本血液学会 日本内科学会 専門医資格 総合内科専門医 血液専門医 学位 京都大学大学院医学研究科 博士(医学) |
臨床検査技師、臨床工学技士、薬剤師
治療一覧
保険診療
CAR-治療(キムリア)治験
臨床研究推進センターはこちら企業・アカデミアの方へ
事業内容
病院における治験・治療の実施にあたり各診療科・部門との院内調整
- 企業・外部医療機関との調整、相談受付
- 細胞治療における細胞調製、保管管理
- 介入患者のフォローアップ支援 臨床推進研究センターとの連携
※当院では細胞製剤の製造業務は行なっておりません
一般の方へ
細胞治療ご希望の方
当センターでは個々の疾患に対する相談は受け付けておりません。
細胞治療および再生医療はすべての疾患に応用可能なわけではありません。
現在の一般治療と比べての優劣は病気の種類、症状、進行具合により様々です。個々の疾患に関する相談は各主治医にお問い合わせください。