プルヴィクトによる前立腺癌治療
当院はプルヴィクト静注(ノバルティスファーマ株式会社)を用いた前立腺がんの治療を2026年1月より開始予定です。プルヴィクトは、前立腺がん細胞に集まる化合物(リガンド)に治療用放射性核種(放射性同位元素 ルテチウム-177)を組み合わせた、新しい前立腺がんの治療薬です。
プルヴィクトによる放射性リガンド療法とは
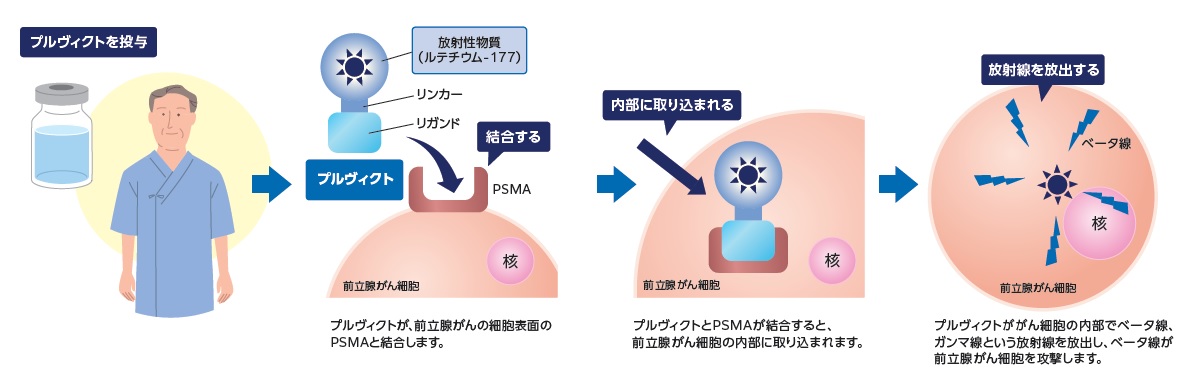
通常の放射線療法(体の外から放射線を当てる治療)とは異なり、薬を静脈から注射し、薬が体の中で放射線を出してがん細胞を攻撃する治療です。
プルヴィクトは、前立腺がん細胞の表面に多く見られる目印「PSMA(ピー・エス・エム・エー)」に結合する薬(リガンド)に、放射性物質(ルテチウム-177)が結びついたものです。
この薬はおもにPSMAを持つ前立腺がん細胞に結合し、結合した近くで放射線(ベータ線)を出してがん細胞を壊します。正常な細胞には結合しにくいため、周囲への影響をできるだけ少なくできると期待されます(まったく影響がないわけではありません)。
ベータ線が体内で届く距離は最大2.2mm(平均1mm未満)と短く、がん細胞周囲の正常な細胞への影響は少ないと考えられます。
プルヴィクト治療の対象となる方
- 対象は、PSMA(前立腺がん細胞の表面に多い”目印”)が陽性で、がんが他の臓器や骨などに広がっている「転移性去勢抵抗性前立腺がん(mCRPC)」の方です。
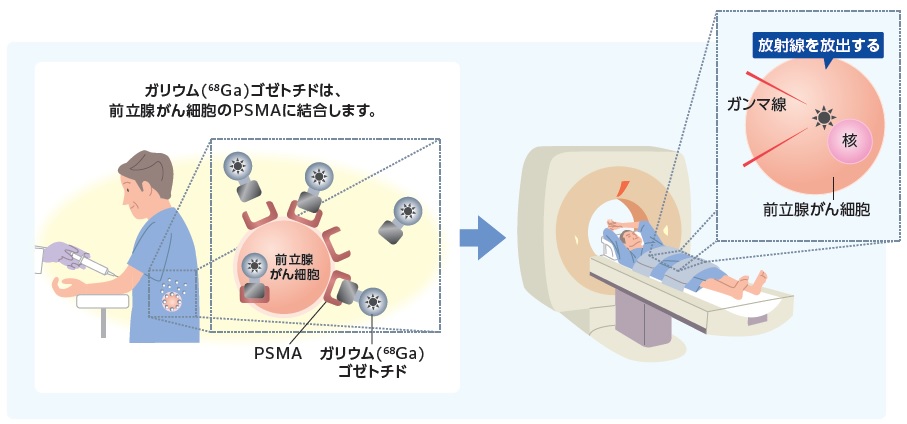
- PSMAが陽性かどうかを、事前に「PSMA-PET検査」(PSMAの有無を画像で確認する検査)を行い、PSMAが陽性であることを確認します。
- また、これまでの治療歴として、次のいずれかに当てはまる方が対象です。
- (1) 新規アンドロゲン受容体シグナル阻害薬(ARSI)を1種類使用し、化学療法(抗癌剤治療)は未実施の方
- (2) ARSIを使用し、さらに化学療法も受けた方
プルヴィクトによる治療のスケジュール
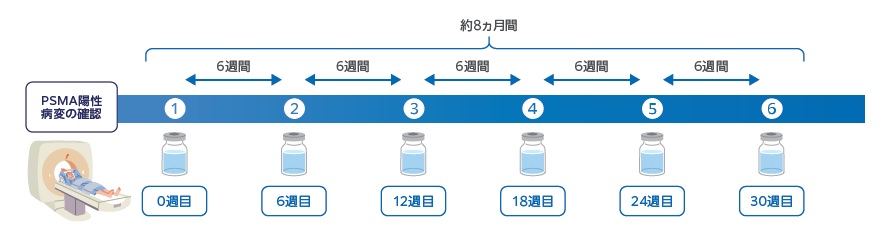
プルヴィクトは、6週間ごとに最大6回注射します。注射した日から数日間、入院する必要があります。すべての治療期間は、約8か月間です。
受診・相談方法
プルヴィクトによる放射性リガンド療法をご希望の方は、当院の泌尿器科外来を受診し、ご相談ください。
かかりつけの病院で本治療を行っていない場合は、主治医にご相談のうえ、当院への紹介をご依頼ください。
患者さん個人からのご予約は受け付けておりません。
*受診後、治療の適応は診察や検査結果をふまえて医師が総合的に判断します。
*本治療に対応した病棟が限られるため、本治療の適応がある場合でも、治療開始までお待ちいただくことがあります。